Altered neuronal mitochondrial coenzyme A synthesis in neurodegeneration with brain iron accumulation caused by abnormal processing, stability, and catalytic activity of mutant pantothenate kinase 2 - PubMed (original) (raw)
Altered neuronal mitochondrial coenzyme A synthesis in neurodegeneration with brain iron accumulation caused by abnormal processing, stability, and catalytic activity of mutant pantothenate kinase 2
Paul T Kotzbauer et al. J Neurosci. 2005.
Abstract
Mutations in the pantothenate kinase 2 (PANK2) gene have been identified in patients with neurodegeneration with brain iron accumulation (NBIA; formerly Hallervorden-Spatz disease). However, the mechanisms by which these mutations cause neurodegeneration are unclear, especially given the existence of multiple pantothenate kinase genes in humans and multiple PanK2 transcripts with potentially different subcellular localizations. We demonstrate that PanK2 protein is localized to mitochondria of neurons in human brain, distinguishing it from other pantothenate kinases that do not possess mitochondrial-targeting sequences. PanK2 protein translated from the most 5' start site is sequentially cleaved at two sites by the mitochondrial processing peptidase, generating a long-lived 48 kDa mature protein identical to that found in human brain extracts. The mature protein catalyzes the initial step in coenzyme A (CoA) synthesis but displays feedback inhibition in response to species of acyl CoA rather than CoA itself. Some, but not all disease-associated point mutations result in significantly reduced catalytic activity. The most common mutation, G521R, results in marked instability of the intermediate PanK2 isoform and reduced production of the mature isoform. These results suggest that NBIA is caused by altered neuronal mitochondrial lipid metabolism caused by mutations disrupting PanK2 protein levels and catalytic activity.
Figures
Figure 1.
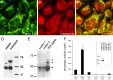
Localization of a 48 kDa PanK2 protein to neuronal mitochondria in human brain.A, Multiple PanK2 antibodies specifically recognize a 48 kDa protein in human brain. Samples of frozen cortex from two different pathologically normal postmortem cases (lanes 1 and 2) were extracted in RIPA buffer and analyzed by Western blot with each of the indicated PanK2 antibodies, revealing a commonly recognized 48 kDa band. B-I, Immunohistochemical analysis demonstrates prominent neuronal expression of PanK2 protein in human brain. Antibodies PanK2 549, 550, and 25.1 were used with brown DAB chromagen detection of bound antibody and produced consistent staining patterns throughout the brain. Prominent staining of neuronal cytoplasm and proximal dendrites (arrowheads) was found in multiple brain areas, including the cortex (B, C), globus pallidus (D, E), nucleus basalis of Meynert (F), pontine nuclei in the basis pontis (G, H), and putamen (I). J-M, Immunoelectron microscopy demonstrates localization of PanK2 protein to neuronal mitochondria in human brain. Sections of human motor cortex were stained with PanK2 549 as described above, developed with silver enhancement of DAB chromagen, and processed for EM as described. Illustrative photographs show numerous mitochondria within a large cortical neuron that were strongly labeled with silver grains specific for PanK2 staining. Similar mitochondrial staining was observed in globus pallidus neurons (data not shown). Boxes in J indicate fields shown at higher magnification in K-M. Scale bars: (in B) B, D, F, H, I, 50 μm; C, E, 20 μm; G, 500 μm; (in K) J, 2.5 μm; K-M, 0.5 μm.
Figure 2.
Transfection of 293 cells with PanK2 cDNA produces multiple PanK2 isoforms that are targeted to the mitochondria and catalyze pantothenate phosphorylation. A-C, Immunofluorescence staining demonstrates colocalization of PanK2 and mitotrackerred staining. Stably transfected 293 cells were stained with PanK2 (A) and mitotracker red CMXros (B). Merged red and green images (C) demonstrate colocalization of staining. D, Multiple PanK2 isoforms are present in extracts of transfected 293 cells. Extracts of transiently transfected and stably transfected 293 cells were analyzed by Western blot using PanK2 549 antibody. Although transiently transfected cells displayed variable subcellular localization of PanK2 (data not shown) and contained predominantly 63 and 48 kDa isoforms, stably transfected cells contained the 48 kDa isoform in combination with a 59 kDa isoform. E, The 48 kDa PanK2 isoform is present in human brain and stably transfected 293 cells. Extracts from PanK2-transfected 293 cells and from human cortex (cases 1 and 2) were analyzed by Western blot analysis with PanK2 25.1. ECL detection with a longer exposure time reveals 63, 59, and 48 kDa isoforms in 293 cells. The 48 kDa isoform is present in human brain as well as 293 cells. F, Mitochondrial extracts of PanK2-transfected cells catalyze the phosphorylation of pantothenate in vitro. Subcellular fractions of 293 cells were assayed for pantothenate kinase activity in vitro. Enzymatic activity corresponded to the predominantly mitochondrial distribution of PanK2 protein observed by Western blot analysis of subcellular fractions (inset). A similar distribution profile was observed by Western blot analysis for the mitochondrial protein pyruvate dehydrogenase (data not shown).
Figure 3.
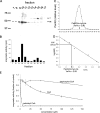
Pulse-chase 35Slabeling of PanK2-myc-his-transfected 293 cells demonstrates sequential proteolytic processing of newly synthesized PanK2 protein. The 293 cells were labeled with 35S-methionine for 30 min and then chased with unlabeled amino acids. A, Cells were harvested at the indicated times after the initiation of labeling and analyzed by immunoprecipitation with PanK2 549 followed by SDS-PAGE. Migration of molecular weight markers is indicated on the left. The positions of the three major PanK2 isoforms are indicated on the right (note the 3 kDa upward shift in molecular weight caused by myc-his tag). A 40 kDa band was inconsistently observed and may arise from proteolysis of the mature isoform during sample processing. B, Quantitation of relative amounts of labeled isoforms at each time point demonstrates the temporal relationship of PanK2 isoforms. PanK2p is the first isoform to appear during the labeling period, and its levels rapidly decline during the chase period as levels of PanK2i rise. The PanK2m isoform rises in a delayed manner corresponding to the decline of the PanK2 intermediate isoform. Similar results were obtained using multiple clonal lines in more than three separate experiments with both native and myc-his-tagged human PanK2.
Figure 4.
N-terminal sequence analysis of PanK2i and PanK2m identifies cleavage sites, implicating the mitochondrial processing peptidase in both cleavage steps. PanK2 protein purified from stably transfected 293 cells was resolved on SDS-PAGE and transferred to a PVDF membrane. Bands corresponding to those indicated on a Coomassie-stained gel (A) were excised and subjected to amino acid sequencing. B, Predicted cleavage site in PanK2p based on N-terminal amino acid sequence obtained for PanK2i. An arginine residue at position -2 relative to the cleavage site is predicted to direct cleavage by MPP. C, Predicted cleavage site in PanK2i based on N-terminal sequence of PanK2m. An arginine residue at position -3 relative to the cleavage site is predicted to direct cleavage by MPP. A less abundant peptide with sequence beginning at residue 143 was also detected during analysis of mature PanK2. D, Schematic model of PanK2 protein indicating the position of cleavage sites in relation to predicted catalytic and regulatory domains of the mature enzyme. The position of NBIA-associated point mutations is indicated with amino acid numbers corresponding to the ATG start site rather than the CTG start site (Hayflick et al., 2003).
Figure 5.
Biochemical characterization of PanK2 protein. A, B, Chromatographic separation of PanK2i and PanK2m demonstrates catalytic activity for both isoforms. Mitochondrial fractions from stably transfected 293 cells were fractionated using a SP Sepharose cation exchange column, and fractions were analyzed by Western blot analysis (A) as well as by immunoprecipitation and enzymatic assay (B). Two peaks in catalytic activity correspond to peaks in PanK2m and PanK2i. C, D, Gel filtration analysis of PanK2 indicates that mature PanK2 is a homodimer. Purified PanK2-myc-his produced in 293 cells was fractionated using a Superose 6 gel filtration column. C, Levels of PanK2 protein in individual fractions were determined by quantitative Western blot using 125I detection. D, Analysis of PanK2 elution time in relation to molecular weight standards results in a predicted molecular weight of 109 kDa, approximately twice the molecular weight of the myc-his-tagged PanK2 protein. E, CoA esters regulate the enzymatic activity of PanK2. Pantothenate kinase enzymatic activity was measured in the presence of various concentrations of dephospho-CoA, CoA, and palmitoyl CoA. As shown on the graph, PanK2 enzymatic activity was negatively regulated by palmitoyl CoA much more than CoA, and displayed little sensitivity to dephospho-CoA. Error bars indicate SD (n = 3). Similar results were obtained in three independent analyses for each compound.
Figure 6.
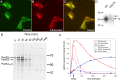
Altered stability and processing for G521R mutant PanK2. A-C, G521R mutant PanK2 is targeted to the mitochondria in stably transfected 293 cells. Staining with PanK2 549 is shown in green (A), and staining with mitotracker red CMXros is shown in red (B). The merged image (C) demonstrates colocalization similar to that seen for wild-type PanK2. D, The G521R mutation results in a reduced steady-state ratio of mature to intermediate isoforms in 293 cells. Western blot analysis of 293 cells stably transfected with wild-type or G521R mutant PanK2-myc-his demonstrates marked reduction in the mature isoform relative to the intermediate isoform with the G521R mutation, in contrast to wild-type PanK2, in which the mature isoform is ∼10-fold more abundant than the intermediate isoform. E, Pulse-chase labeling of G521R PanK2-myc-his-transfected 293 cells reveals altered stability and processing of G521R compared with WT. 293 cells were labeled and analyzed by immunoprecipitation and SDS-PAGE as in Figure 3. F, Levels of each isoform for mutant and WT PanK2 were normalized to the total amount of labeled PanK2 (all three isoforms) present at the end of the labeling period (30 min time point) and graphed over time. Similar results were obtained in two independent experiments.
Figure 7.
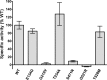
Multiple disease-associated point mutations disrupt PanK2 catalytic activity. QBI 293 cells were transiently transfected with expression plasmids containing wild-type PanK2 or PanK2 containing various disease-associated point mutations. Extracts of transfected cells were prepared, and the concentration of PanK2 protein in each extract was determined by quantitative Western blot using PanK2 549 antibody and 125I protein A detection of bound primary antibody. After normalizing the volumes of extract to equalize total PanK2 protein, catalytic activity was determined by pantothenate kinase enzymatic assay. Because QBI 293 cells produce endogenous panthothenate kinases, catalytic activity was measured for corresponding volumes of vector-transfected QBI 293 cells and subtracted from values obtained for each of the PanK2-transfected cell extracts. Error bars indicate 95% confidence intervals for each mean (n = 3). Similar results were obtained in two independent experiments.
Similar articles
- Pantothenate Rescues Iron Accumulation in Pantothenate Kinase-Associated Neurodegeneration Depending on the Type of Mutation.
Álvarez-Córdoba M, Fernández Khoury A, Villanueva-Paz M, Gómez-Navarro C, Villalón-García I, Suárez-Rivero JM, Povea-Cabello S, de la Mata M, Cotán D, Talaverón-Rey M, Pérez-Pulido AJ, Salas JJ, Pérez-Villegas EM, Díaz-Quintana A, Armengol JA, Sánchez-Alcázar JA. Álvarez-Córdoba M, et al. Mol Neurobiol. 2019 May;56(5):3638-3656. doi: 10.1007/s12035-018-1333-0. Epub 2018 Sep 1. Mol Neurobiol. 2019. PMID: 30173408 - Down regulation of the expression of mitochondrial phosphopantetheinyl-proteins in pantothenate kinase-associated neurodegeneration: pathophysiological consequences and therapeutic perspectives.
Álvarez-Córdoba M, Talaverón-Rey M, Villalón-García I, Povea-Cabello S, Suárez-Rivero JM, Suárez-Carrillo A, Munuera-Cabeza M, Salas JJ, Sánchez-Alcázar JA. Álvarez-Córdoba M, et al. Orphanet J Rare Dis. 2021 May 5;16(1):201. doi: 10.1186/s13023-021-01823-3. Orphanet J Rare Dis. 2021. PMID: 33952316 Free PMC article. - Biochemical properties of human pantothenate kinase 2 isoforms and mutations linked to pantothenate kinase-associated neurodegeneration.
Zhang YM, Rock CO, Jackowski S. Zhang YM, et al. J Biol Chem. 2006 Jan 6;281(1):107-14. doi: 10.1074/jbc.M508825200. Epub 2005 Nov 3. J Biol Chem. 2006. PMID: 16272150 - Unraveling the Hallervorden-Spatz syndrome: pantothenate kinase-associated neurodegeneration is the name.
Hayflick SJ. Hayflick SJ. Curr Opin Pediatr. 2003 Dec;15(6):572-7. doi: 10.1097/00008480-200312000-00005. Curr Opin Pediatr. 2003. PMID: 14631201 Review. - Neurodegeneration with brain iron accumulation.
Gregory A, Hayflick SJ. Gregory A, et al. Folia Neuropathol. 2005;43(4):286-96. Folia Neuropathol. 2005. PMID: 16416393 Free PMC article. Review.
Cited by
- Neurodegeneration with brain iron accumulation: an overview.
Tonekaboni SH, Mollamohammadi M. Tonekaboni SH, et al. Iran J Child Neurol. 2014 Fall;8(4):1-8. Iran J Child Neurol. 2014. PMID: 25657764 Free PMC article. Review. - Pathophysiology and treatment of neurodegeneration with brain iron accumulation in the pediatric population.
Schneider SA, Zorzi G, Nardocci N. Schneider SA, et al. Curr Treat Options Neurol. 2013 Oct;15(5):652-67. doi: 10.1007/s11940-013-0254-5. Curr Treat Options Neurol. 2013. PMID: 23888388 - Overdosing on iron: Elevated iron and degenerative brain disorders.
D'Mello SR, Kindy MC. D'Mello SR, et al. Exp Biol Med (Maywood). 2020 Oct;245(16):1444-1473. doi: 10.1177/1535370220953065. Epub 2020 Sep 2. Exp Biol Med (Maywood). 2020. PMID: 32878460 Free PMC article. Review. - A Potential Citrate Shunt in Erythrocytes of PKAN Patients Caused by Mutations in Pantothenate Kinase 2.
Werning M, Dobretzberger V, Brenner M, Müllner EW, Mlynek G, Djinovic-Carugo K, Baron DM, Fragner L, Bischoff AT, Büchner B, Klopstock T, Weckwerth W, Salzer U. Werning M, et al. Biomolecules. 2022 Feb 18;12(2):325. doi: 10.3390/biom12020325. Biomolecules. 2022. PMID: 35204826 Free PMC article. - Drug Drop Test: How to Quickly Identify Potential Therapeutic Compounds for Mitochondrial Diseases Using Yeast Saccharomyces cerevisiae.
Magistrati M, Gilea AI, Gerra MC, Baruffini E, Dallabona C. Magistrati M, et al. Int J Mol Sci. 2023 Jun 27;24(13):10696. doi: 10.3390/ijms241310696. Int J Mol Sci. 2023. PMID: 37445873 Free PMC article. Review.
References
- Begley TP, Kinsland C, Strauss E (2001) The biosynthesis of coenzyme A in bacteria. Vitam Horm 61: 157-171. - PubMed
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 3: 1301-1306. - PubMed
- Betts J, Lightowlers RN, Turnbull DM (2004) Neuropathological aspects of mitochondrial DNA disease. Neurochem Res 29: 505-511. - PubMed
- Bindoff LA, Birch-Machin M, Cartlidge NE, Parker Jr WD, Turnbull DM (1989) Mitochondrial function in Parkinson's disease. Lancet 2: 49. - PubMed
- Branda SS, Cavadini P, Adamec J, Kalousek F, Taroni F, Isaya G (1999) Yeast and human frataxin are processed to mature form in two sequential steps by the mitochondrial processing peptidase. J Biol Chem 274: 22763-22769. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases