Multiple protein interactions involving proposed extracellular loop domains of the tight junction protein occludin - PubMed (original) (raw)
Multiple protein interactions involving proposed extracellular loop domains of the tight junction protein occludin
Asma Nusrat et al. Mol Biol Cell. 2005 Apr.
Abstract
Occludin is a tetraspan integral membrane protein in epithelial and endothelial tight junction (TJ) structures that is projected to have two extracellular loops. We have used peptides emulating central regions of human occludin's first and second loops, termed O-A:101-121 and O-B:210-228, respectively, to examine potential molecular interactions between these two regions of occludin and other TJ proteins. A superficial biophysical assessment of A:101-121 and O-B:210-228 showed them to have dissimilar solution conformation characteristics. Although O-A:101-121 failed to strongly interact with protein components of the human epithelial intestinal cell line T84, O-B:210-228 selectively associated with occludin, claudin-one and the junctional adhesion molecule (JAM)-A. Further, the presence of O-B:210-228, but not O-A:101-121, impeded the recovery of functional TJ structures. A scrambled peptide sequences of O-B:210-228 failed to influence TJ assembly. These studies demonstrate distinct properties for these two extracellular segments of the occludin protein and provide an improved understanding of how specific domains of occludin may interact with proteins present at TJ structures.
Figures
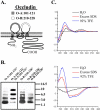
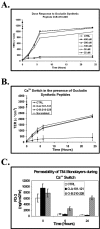
Figure 1.
Properties of occludin synthetic peptides. (A) Cartoon of human occludin demonstrating sequence locations emulated by O-A:101–121 and O-B:210–228 peptides (detailed in Table 1). (B) Homologous peptide association studies performed with 20 μM bait peptide incubated in HBSS with Ca2+ and Mg2+ (HBSS+) in the presence or absence of 200 μM corresponding standard peptide. (C) Circular dichroism (CD) spectra of 0.2 mg standard peptide dissolved in H2O, sodium dodecylsulfate (SDS) micelles, or 92% trifluoroethanol (TFE).
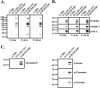
Figure 2.
Association of occludin bait peptides with cell proteins. Divalent cation depletion was used to dissemble tight junction (TJ) structures of polarized, confluent T84 cell monolayers. Bait peptide (200 μM O-A:101–121* or O-B:210–228*) was added at the time of Ca2+ repletion (T = 0) and monolayers were evaluated 0, 6, or 24 h later. After being washed free of unbound peptide monolayers were lysed. (A) After cell lysis and separation by SDS-PAGE, complete bait peptide labeling was determined by avidin labeling. (B) Monomeric avidin-Sepharose column-captured material was assessed by Western blot analysis. (C) Avidin-captured material was immunoprecipitated (IP) with an antibody recognizing human occludin, separated by SDS-PAGE, and Western-blotted an antibody to occludin. (D) Western blot analysis of IP occludin labeled by O-B:210–228* using antibodies that specifically recognize phospho-serine, phospho-threonine, and phosphotyrosine.
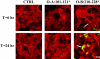
Figure 3.
Localization of occludin peptides. Divalent cation depletion was used to dissemble tight junction (TJ) structures of polarized, confluent T84 cell monolayers. Bait peptide (200 μM O-A:101–121* or O-B: 210–228*) or media used for peptide addition (CTRL) was added at the time of Ca2+ repletion. After a 6- or 24-h incubation, monolayers were washed free of unbound peptide, fixed with 3.7% paraformaldehyde, permeabilized with 0.2% Triton X-100, and prepared for fluorescence microscopy. Distribution of bound bait peptides was determined by staining with FITC-conjugated streptavidin (green), whereas Alexa 568-phalloidin was used to highlight the F-actin architecture (red). Scale bar, 10 μm.
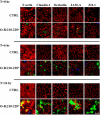
Figure 4.
Effects of bait peptides on TJ architecture. Confluent T84 monolayers were subjected to a Ca2+ switch protocol with 200 μM O-A:101–121*, O-B:210–228*, or control media being added at the time of Ca2+ repletion. Immediately after (T = 0) or after 6- or 24-h incubation, monolayers were washed free of unbound peptide, fixed with ethanol or 3.7% paraformaldehyde, and prepared for fluorescence microscopy. Localization of claudin-1, occludin, JAM-A, and ZO-1 was determined by labeling with protein-specific antibodies that could be recognized by a fluorescently labeled secondary antibody (red). Distribution of bound bait peptides was determined by staining with FITC-conjugated streptavidin (green), Alexa 568-phalloidin as used to highlight the F-actin architecture (red). Scale bar, 10 μm.
Figure 5.
Effects of peptides on TJ function. Divalent cation depletion was used to dissemble tight junction (TJ) structures of polarized, confluent T84 cell monolayers. (A) O-B:210–228 was added to final concentrations from 25 to 400 μM at the time of Ca2+ repletion. Addition of media used for peptide additions was used as a control (CTRL). Transepithelial electrical resistance (TER) measurements were performed over the next 24 h using “chopstick” electrodes. (B) After divalent cation depletion, 200 μM O-A:101–121, O-B:210–228, control (scrambled O-B:210–228 sequence) peptides, or control media was added to T84 monolayers at the time of Ca2+ repletion. TER measurements were made over the next 24 h using chopstick electrodes. (C) After addition of 200 μM O-A:101–121, O-B:210–228, or control media at the time of Ca2+ repletion, paracellular permeability was determined as a rate of 3-kDa fluorescent dextran transport at T = 0, 6, and 24 h.
Similar articles
- Cholesterol depletion alters detergent-specific solubility profiles of selected tight junction proteins and the phosphorylation of occludin.
Lynch RD, Francis SA, McCarthy KM, Casas E, Thiele C, Schneeberger EE. Lynch RD, et al. Exp Cell Res. 2007 Jul 15;313(12):2597-610. doi: 10.1016/j.yexcr.2007.05.009. Epub 2007 May 18. Exp Cell Res. 2007. PMID: 17574235 Free PMC article. - A key claudin extracellular loop domain is critical for epithelial barrier integrity.
Mrsny RJ, Brown GT, Gerner-Smidt K, Buret AG, Meddings JB, Quan C, Koval M, Nusrat A. Mrsny RJ, et al. Am J Pathol. 2008 Apr;172(4):905-15. doi: 10.2353/ajpath.2008.070698. Epub 2008 Mar 18. Am J Pathol. 2008. PMID: 18349130 Free PMC article. - Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins.
Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Kucharzik T, et al. Am J Pathol. 2001 Dec;159(6):2001-9. doi: 10.1016/S0002-9440(10)63051-9. Am J Pathol. 2001. PMID: 11733350 Free PMC article. - Occludin: one protein, many forms.
Cummins PM. Cummins PM. Mol Cell Biol. 2012 Jan;32(2):242-50. doi: 10.1128/MCB.06029-11. Epub 2011 Nov 14. Mol Cell Biol. 2012. PMID: 22083955 Free PMC article. Review. - Occludin protein family: oxidative stress and reducing conditions.
Blasig IE, Bellmann C, Cording J, Del Vecchio G, Zwanziger D, Huber O, Haseloff RF. Blasig IE, et al. Antioxid Redox Signal. 2011 Sep 1;15(5):1195-219. doi: 10.1089/ars.2010.3542. Epub 2011 May 5. Antioxid Redox Signal. 2011. PMID: 21235353 Review.
Cited by
- Occludin independently regulates permeability under hydrostatic pressure and cell division in retinal pigment epithelial cells.
Phillips BE, Cancel L, Tarbell JM, Antonetti DA. Phillips BE, et al. Invest Ophthalmol Vis Sci. 2008 Jun;49(6):2568-76. doi: 10.1167/iovs.07-1204. Epub 2008 Feb 8. Invest Ophthalmol Vis Sci. 2008. PMID: 18263810 Free PMC article. - Critical role of tight junctions in drug delivery across epithelial and endothelial cell layers.
González-Mariscal L, Nava P, Hernández S. González-Mariscal L, et al. J Membr Biol. 2005 Sep;207(2):55-68. doi: 10.1007/s00232-005-0807-y. J Membr Biol. 2005. PMID: 16477528 Review. - Host Invasion by Pathogenic Amoebae: Epithelial Disruption by Parasite Proteins.
Betanzos A, Bañuelos C, Orozco E. Betanzos A, et al. Genes (Basel). 2019 Aug 14;10(8):618. doi: 10.3390/genes10080618. Genes (Basel). 2019. PMID: 31416298 Free PMC article. Review. - Brain endothelial cell-cell junctions: how to "open" the blood brain barrier.
Stamatovic SM, Keep RF, Andjelkovic AV. Stamatovic SM, et al. Curr Neuropharmacol. 2008 Sep;6(3):179-92. doi: 10.2174/157015908785777210. Curr Neuropharmacol. 2008. PMID: 19506719 Free PMC article. - Occludin oligomeric assembly at tight junctions of the blood-brain barrier is disrupted by peripheral inflammatory hyperalgesia.
McCaffrey G, Seelbach MJ, Staatz WD, Nametz N, Quigley C, Campos CR, Brooks TA, Davis TP. McCaffrey G, et al. J Neurochem. 2008 Sep;106(6):2395-409. doi: 10.1111/j.1471-4159.2008.05582.x. Epub 2008 Jul 21. J Neurochem. 2008. PMID: 18647175 Free PMC article.
References
- Aoki, N., Ito, K., and Ito, M. (1997). Isolation and characterization of mouse high-glycine/tyrosine proteins. J. Biol. Chem. 272, 30512–30518. - PubMed
- Balda, M. S., Whitney, J. A., Flores, C., Gonzalez, S., Cereijido, M., and Matter, K. (1996). Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 134, 1031–1049. - PMC - PubMed
- Colegio, O. R., Van Itallie, C., Rahner, C., and Anderson, J. M. (2003). Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am. J. Physiol. Cell Physiol. 284, C1346–C1354. - PubMed
- Colegio, O. R., Van Itallie, C. M., McCrea, H. J., Rahner, C., and Anderson, J. M. (2002). Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am. J. Physiol. Cell Physiol. 283, C142–C147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous