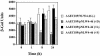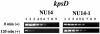Down-regulation of the kps region 1 capsular assembly operon following attachment of Escherichia coli type 1 fimbriae to D-mannose receptors - PubMed (original) (raw)
Down-regulation of the kps region 1 capsular assembly operon following attachment of Escherichia coli type 1 fimbriae to D-mannose receptors
William R Schwan et al. Infect Immun. 2005 Feb.
Abstract
A differential-display PCR procedure identified the capsular assembly gene kpsD after Escherichia coli type 1 fimbrial binding to mannose-coated Sepharose beads. Limiting-dilution reverse-transcribed PCRs confirmed down-regulation of the kpsD gene, and Northern blot and lacZ fusion analyses showed down-regulation of the kpsFEDUCS region 1 operon. KpsD protein levels fell, and an agglutination test showed less K capsular antigen on the surface following the bacterial ligand-receptor interaction. These data show that binding of type 1 fimbriae (pili) to d-mannose receptors triggers a cross talk that leads to down-regulation of the capsule assembly region 1 operon in uropathogenic E. coli.
Figures
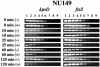
FIG. 1.
Quantitative determination of kpsD and ftsZ transcript levels by LD-RT-PCR analysis. The cDNAs generated from strain NU149 cells mixed with plain Sepharose or
d
-mannose-coated Sepharose beads were examined at 0, 10, 25, 60, and 120 min. The KpsD1-KpsD2 and ECFtsZ1-ECFtsZ2 primer pairs were used to amplify serially twofold-diluted cDNAs targeting kpsD (367-bp product) and ftsZ (302-bp product) transcripts, respectively. The PCR amplifications were done a minimum of three times. All PCR products were electrophoresed on 1.5% agarose gels. The following dilutions of cDNAs were used: undiluted (lane 1), 1:2 (lane 2), 1:4 (lane 3), 1:8 (lane 4), 1:16 (lane 5), 1:32 (lane 6), 1:64 (lane 7), 1:128 (lane 8), and 1:256 (lane 9).
FIG. 2.
Regulation of the kpsFEDUCS promoter following attachment to plain Sepharose (−),
l
-mannose-coated Sepharose (+L),
d
-mannose-coated Sepharose (+D), or plain Sepharose plus 50 mM free
d
-mannose (+F). The assays were done using a kpsFEDUCS promoter fused to lacZYA as a transcriptional fusion on a single-copy-number plasmid placed in strain AAEC189. The β-galactosidase (β-Gal) activity is expressed in Miller units; means ± standard deviations are indicated from three separate runs.
FIG. 3.
Quantitative determination of kpsD transcript levels in strains NU14 (wild type) and NU14-1 (fimH mutant) by LD-RT-PCR analysis. The cDNAs generated from strain NU14 or NU14-1 cells mixed with
d
-mannose-coated Sepharose beads at 0 and 120 min were examined. The KpsD1-KpsD2 primer pair was used to amplify serially twofold-diluted cDNAs targeting kpsD (367-bp product) transcripts. The PCR amplifications were done a minimum of three times. All PCR products were electrophoresed on 1.5% agarose gels. The following dilutions of cDNAs were used: undiluted (lane 1), 1:2 (lane 2), 1:4 (lane 3), 1:8 (lane 4), 1:16 (lane 5), 1:32 (lane 6), 1:64 (lane 7), 1:128 (lane 8), and 1:256 (lane 9).
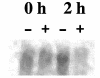
FIG. 4.
Northern hybridization analysis of transcription of the kpsFEDUCS operon from total RNAs isolated from NU149 cells mixed with plain Sepharose (−) or
d
-mannose-coated Sepharose (+) beads after 0 and 2 h of exposure. Ten micrograms of total RNA from NU149 cells mixed with plain Sepharose beads or
d
-mannose-coated Sepharose beads was probed with a kpsD DNA fragment. After 6 days, the blot was developed with a phosphorimager and the amounts of kpsFEDUCS RNA (approximate size, 7.9 kb) were compared between lanes by using ImageQuant 5.2 software.
FIG. 5.
Enzyme-linked immunosorbent assay analysis of the KpsD protein from strain NU149 cells mixed with plain or
d
-mannose-coated Sepharose beads for 0, 8, and 24 h. Optical densities at 405 nm (O.D.405) were determined, and means ± standard deviations are indicated from three runs. (−), plain Sepharose; (+),
d
-mannose-coated Sepharose.
FIG. 6.
Directagen analysis of K1 capsular expression in strain NU14. Bacterial cells were mixed with plain Sepharose (−) or
d
-mannose-coated Sepharose (+) beads and assayed after 0 and 24 h. White clumps of bacteria indicate a positive response.
Similar articles
- Peptidoglycan Association of Murein Lipoprotein Is Required for KpsD-Dependent Group 2 Capsular Polysaccharide Expression and Serum Resistance in a Uropathogenic Escherichia coli Isolate.
Diao J, Bouwman C, Yan D, Kang J, Katakam AK, Liu P, Pantua H, Abbas AR, Nickerson NN, Austin C, Reichelt M, Sandoval W, Xu M, Whitfield C, Kapadia SB. Diao J, et al. mBio. 2017 May 23;8(3):e00603-17. doi: 10.1128/mBio.00603-17. mBio. 2017. PMID: 28536290 Free PMC article. - The major subunit of Escherichia coli type 1 fimbriae is not required for D-mannose-specific adhesion.
Klemm P, Krogfelt KA, Hedegaard L, Christiansen G. Klemm P, et al. Mol Microbiol. 1990 Apr;4(4):553-9. doi: 10.1111/j.1365-2958.1990.tb00623.x. Mol Microbiol. 1990. PMID: 1972261 - Altered Regulation of the Diguanylate Cyclase YaiC Reduces Production of Type 1 Fimbriae in a Pst Mutant of Uropathogenic Escherichia coli CFT073.
Crépin S, Porcheron G, Houle S, Harel J, Dozois CM. Crépin S, et al. J Bacteriol. 2017 Nov 14;199(24):e00168-17. doi: 10.1128/JB.00168-17. Print 2017 Dec 15. J Bacteriol. 2017. PMID: 28924030 Free PMC article. - Molecular structure of adhesin domains in Escherichia coli fimbriae.
Westerlund-Wikström B, Korhonen TK. Westerlund-Wikström B, et al. Int J Med Microbiol. 2005 Oct;295(6-7):479-86. doi: 10.1016/j.ijmm.2005.06.010. Int J Med Microbiol. 2005. PMID: 16238022 Review. - Biosynthesis and assembly of capsular polysaccharides in Escherichia coli.
Whitfield C. Whitfield C. Annu Rev Biochem. 2006;75:39-68. doi: 10.1146/annurev.biochem.75.103004.142545. Annu Rev Biochem. 2006. PMID: 16756484 Review.
Cited by
- Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: recent reports.
Sarowska J, Futoma-Koloch B, Jama-Kmiecik A, Frej-Madrzak M, Ksiazczyk M, Bugla-Ploskonska G, Choroszy-Krol I. Sarowska J, et al. Gut Pathog. 2019 Feb 21;11:10. doi: 10.1186/s13099-019-0290-0. eCollection 2019. Gut Pathog. 2019. PMID: 30828388 Free PMC article. Review. - Transcriptional response of E. coli upon FimH-mediated fimbrial adhesion.
Bhomkar P, Materi W, Semenchenko V, Wishart DS. Bhomkar P, et al. Gene Regul Syst Bio. 2010 Mar 24;4:1-17. doi: 10.4137/grsb.s4525. Gene Regul Syst Bio. 2010. PMID: 20458372 Free PMC article. - Broad-spectrum biofilm inhibition by a secreted bacterial polysaccharide.
Valle J, Da Re S, Henry N, Fontaine T, Balestrino D, Latour-Lambert P, Ghigo JM. Valle J, et al. Proc Natl Acad Sci U S A. 2006 Aug 15;103(33):12558-63. doi: 10.1073/pnas.0605399103. Epub 2006 Aug 7. Proc Natl Acad Sci U S A. 2006. PMID: 16894146 Free PMC article. - Functional Insights From KpfR, a New Transcriptional Regulator of Fimbrial Expression That Is Crucial for Klebsiella pneumoniae Pathogenicity.
Gomes AÉI, Pacheco T, Dos Santos CDS, Pereira JA, Ribeiro ML, Darrieux M, Ferraz LFC. Gomes AÉI, et al. Front Microbiol. 2021 Jan 21;11:601921. doi: 10.3389/fmicb.2020.601921. eCollection 2020. Front Microbiol. 2021. PMID: 33552015 Free PMC article. - A complex transcription network controls the early stages of biofilm development by Escherichia coli.
Prüss BM, Besemann C, Denton A, Wolfe AJ. Prüss BM, et al. J Bacteriol. 2006 Jun;188(11):3731-9. doi: 10.1128/JB.01780-05. J Bacteriol. 2006. PMID: 16707665 Free PMC article. Review. No abstract available.
References
- Abu Kwaik, Y., and L. L. Pederson. 1996. The use of differential display-PCR to isolate and characterize a Legionella pneumophila locus induced during the intracellular infection of macrophages. Mol. Microbiol. 21:543-556. - PubMed
- Arrecubieta, C., T. C. Hammarton, B. Barrett, S. Chareonsudjai, N. Hodson, D. Rainey, and I. S. Roberts. 2001. The transport of group 2 capsular polysaccharides across the periplasmic space in Escherichia coli. J. Biol. Chem. 276:4245-4250. - PubMed
- Bahrani-Mougeot, F. K., S. Pancholi, M. Daoust, and M. S. Donnenberg. 2001. Identification of putative urovirulence genes by subtractive cloning. J. Infect. Dis. 182:S21-S23. - PubMed
- Bahrani-Mougeot, F. K., E. L. Buckles, C. V. Lockatell, J. R. Hebel, D. E. Johnson, C. M. Tang, and M. S. Donnenberg. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 45:1079-1093. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources