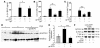The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo - PubMed (original) (raw)
. 2005 Feb;115(2):313-25.
doi: 10.1172/JCI22433.
Tatjana Eigenbrod, Norbert Krug, George T De Sanctis, Michael Hausding, Veit J Erpenbeck, El-Bdaoui Haddad, Hans A Lehr, Edgar Schmitt, Tobias Bopp, Karl-J Kallen, Udo Herz, Steffen Schmitt, Cornelia Luft, Olaf Hecht, Jens M Hohlfeld, Hiroaki Ito, Norihiro Nishimoto, Kazuyuki Yoshizaki, Tadamitsu Kishimoto, Stefan Rose-John, Harald Renz, Markus F Neurath, Peter R Galle, Susetta Finotto
Affiliations
- PMID: 15668741
- PMCID: PMC544603
- DOI: 10.1172/JCI22433
The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo
Aysefa Doganci et al. J Clin Invest. 2005 Feb.
Erratum in
- J Clin Invest. 2005 May;115(5):1388. Lehr, Hans A [added]
Abstract
The cytokine IL-6 acts via a specific receptor complex that consists of the membrane-bound IL-6 receptor (mIL-6R) or the soluble IL-6 receptor (sIL-6R) and glycoprotein 130 (gp130). In this study, we investigated the role of IL-6R components in asthma. We observed increased levels of sIL-6R in the airways of patients with allergic asthma as compared to those in controls. In addition, local blockade of the sIL-6R in a murine model of late-phase asthma after OVA sensitization by gp130-fraction constant led to suppression of Th2 cells in the lung. By contrast, blockade of mIL-6R induced local expansion of Foxp3-positive CD4+CD25+ Tregs with increased immunosuppressive capacities. CD4+CD25+ but not CD4+CD25- lung T cells selectively expressed the IL-6R alpha chain and showed IL-6-dependent STAT-3 phosphorylation. Finally, in an in vivo transfer model of asthma in immunodeficient Rag1 mice, CD4+CD25+ T cells isolated from anti-IL-6R antibody-treated mice exhibited marked immunosuppressive and antiinflammatory functions. IL-6 signaling therefore controls the balance between effector cells and Tregs in the lung by means of different receptor components. Furthermore, inhibition of IL-6 signaling emerges as a novel molecular approach for the treatment of allergic asthma.
Figures
Figure 1
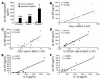
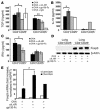
sIL-6R is increased in BALF of asthmatic patients as compared to control subjects, and its levels correlate with the number of IL-5–producing CD4+ T cells in BALF after allergen challenge. (A) sIL-6R was measured before (untreated) and 24 hours after allergen or saline challenge in control subjects (gray bars) or in subjects with asthma (black bars). In the asthmatic patients, sIL-6R levels were increased at baseline and were further increased after allergen challenge. *P < 0.05; ***P < 0.001. (B–D) In these patients, a positive correlation was found between the levels of sIL-6R and the number of CD4+ T cells (r = 0.9027; P < 0.0001) after allergen challenge (C). Furthermore, the value of sIL-6R positively correlated with the number of CD4+ T cells producing IL-5 in BALF (r = 0.9171; P = 0.0001) (D), while a lower correlation was found between sIL-6R and the number of eosinophils (Eos.) in BALF (r = 0.6059; P = 0.0282) (B). Furthermore, the value of sIL-6R in the airways of asthmatic subjects after allergen challenge correlates positively with the respective sIL-6R of IL-5 (E) and IL-13 (F) in BALF.
Figure 2
Local blockade of sIL-6R by gp130-Fc downregulates IL-4, IL-5, and IL-13 levels and reduces GATA-3 expression in experimental asthma. BALB/c mice were sensitized and challenged with OVA whereas control mice were given saline. Some OVA-sensitized mice received additional treatment with gp130-Fc to block sIL-6R function in vivo, as indicated. gp130-Fc treatment was associated with a significant decrease in IL-4 (A), IL-5 (B), and IL-13 (C) levels in BALF of OVA-sensitized mice. *P < 0.05; **P < 0.01; ***P < 0.001. Data represent mean values ± SEM from 5 mice per group. (D) Total lung proteins were isolated from gp130-Fc–treated mice and untreated control mice at day 28 and analyzed by Western blot analysis after immunoblotting with a monoclonal antibody directed against GATA-3. Furthermore, ERK2 expression was determined on the same blot after membrane stripping and incubation with an anti–ERK-2 antibody. Each lane in the Western blot was loaded with 50 μg proteins isolated from different mice (saline, n = 4; OVA, n = 5; OVA + gp130-Fc, n = 5). Quantification of Western blots by densitometry is reported in E and shows decreased GATA-3 expression after i.n. delivery of gp130-Fc in OVA-sensitized and -challenged mice (*P < 0.05; ***P = 0.00056). (F) RT-PCR for GATA-3 and T-bet in 105 lung CD4+ T cells per group after total RNA extraction. Both anti–IL-6R antibody and gp130-Fc treatment led to upregulation of T-bet, while GATA-3 remained unchanged in lung OVA-specific CD4+ T cells.
Figure 3
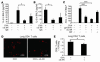
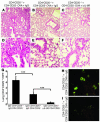
Antiinflammatory mechanism of local treatment with anti–IL-6R antibodies. Blockade of IL-6R led to a downregulation of IL-4 (P = 0.05) (A), and, at higher doses (100 μg/day), of IL-5 (P = 0.029) (B) in the lungs of treated mice. These findings were accompanied by downregulation of the total number of eosinophils (P = 0.0002) in BALF (C) and CD4+ cells (P = 0.05) in the airways of treated mice (D and E). (D) CD4+ lung cells were stained by using monoclonal anti-CD4 antibodies (BD), and immunohistochemistry was performed as previously described (34). Pictures were taken with an Olympus inverted microscope connected to a digital camera. Original magnification, ×400. (E) The number of CD4+ lung cells obtained from 1 lung after CD4 isolation is reported for different groups. *P < 0.05; ***P < 0.001.
Figure 4
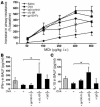
Blockade of mIL-6R through i.n. application of anti–IL-6R antibodies ameliorates AHR and induces IFN-γ and IL-10 levels in BALF in a mouse model of asthma after OVA sensitization. (A) Eight to 10 BALB/c mice per group were sensitized to OVA-alum (OVA-sensitized mice) followed by local treatment with OVA alone or treatment with OVA plus either gp130-Fc, IgG, or anti–IL-6R antibody. Control mice were sensitized with saline-alum and exposed to saline aerosol (saline). Transpulmonary resistance was performed 24 hours after the last local treatment in all mice, as specified in Methods. Dose-response curves to MCh were obtained after administering indicated doses of intravenous MCh. OVA-sensitized mice reacted with an increase of airway resistance at low doses of MCh as compared to that of mice given saline. Anti–IL-6R–treated, OVA-sensitized mice were more protected from the development of AHR compared to untreated (P = 0.049) or IgG-treated, OVA-sensitized mice. Moreover, blockade of sIL-6R by gp130-Fc was less effective compared to anti–IL-6R antibody treatment. (B and C) Local anti–IL-6R antibody treatment induced significant release of IFN-γ (P = 0.048) (B) and IL-10 (P = 0.020) (C) in BALF of OVA-senstitized mice as compared to untreated, OVA-sensitized mice (5 < n < 15). Mean values ± SEM are shown. *P < 0.05.
Figure 5
IL-10–producing CD4+ T cells in the lungs of anti–IL-6R antibody–treated mice. (A and B) CD4+ T cells were isolated from the lung of treated or untreated mice and cultured overnight in the presence of anti-CD3 antibodies. CBA was performed on the CD4+ T cell supernatants. CD4+ T cells isolated from the lung of anti–IL-6R antibody–treated mice secreted increased amounts of IL-10 and IFN-γ as compared to those of OVA-sensitized and -challenged, untreated or IgG-treated mice (P = 0.023 and P = 0.013 for IL-10 and IFN-γ, respectively). Levels of the Th2-type chemokine MCP-1 were not upregulated upon anti–IL-6R antibody treatment, however. (C and D) By contrast, lung CD4+ cells isolated from mice treated i.n. with gp130-Fc did not show changes either in IL-10 (C) or IFN-γ production (D).
Figure 6
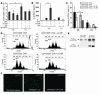
Increased release of IL-10 from Foxp3+ CD4+CD25+ Tregs isolated from the lungs of anti–IL-6R–treated mice. CD4+CD25+ T cells and CD4+CD25– T cells were isolated from lung cells in the different experimental groups, after which cytokine production was analyzed. The purity of the CD4+CD25+ cell population was 95–98% as determined by FACS analysis during cell sorting. (A–C) Lung CD4+CD25+ T cells isolated from OVA-sensitized, anti–IL-6R antibody–treated mice released increased amounts of IL-10 (B) per cell as compared to CD4+CD25+ T cells from OVA-sensitized and -challenged, untreated or OVA-sensitized, IgG-treated mice. In contrast, lung CD4+CD25– T cells produced little IL-10 (B) but more TGF-β (A) and some IFN-γ (C). n = 6. By contrast, CD4+CD25+ isolated from gp130-Fc–treated mice released less IL-10 (B), IFN-γ (C), and TGF-β (A), while the CD4+CD25– isolated from the same mice released the same amount of IL-10 (B) and less TGF-β (A). (D) Expression of Foxp3 on CD4+CD25+ lung T cells upon anti–IL-6R antibody treatment. CD4+CD25+ and CD4+CD25– lung T cells from untreated or anti–IL-6R antibody–treated, OVA-sensitized mice were separated as described above. This was followed by RNA extraction and analysis of Foxp3 or β-actin expression by RT-PCR. (E) Real-time PCR for Foxp3 in CD4+CD25+ and CD4+CD25– cells is reported as the ratio of Foxp3 to HGPRT. Anti–IL-6R antibody treatment led to a significant upregulation of Foxp3 as compared to OVA treatment. This experiment was performed 3 times in duplicate. *P < 0.05; **P < 0.01.
Figure 7
Increased number and augmented immunosuppressive function of CD4+CD25+ T cells in the lungs of anti–IL-6R–treated, OVA-sensitized mice. (A) i.n. but not i.p. anti–IL-6R antibody treatment after OVA sensitization and challenge led to an induction of CD4+CD25+ T cell number in the lung (*P = 0.057). (B) IL-6 levels were increased in BALF of OVA-sensitized mice as compared to those of saline-treated mice. i.p. but not i.n. injection of anti–IL-6R antibodies led to a further increase of IL-6 in the airways. (C) CD4+CD25+ T cells isolated from the lungs of anti–IL-6R antibody–treated (i.n.) mice inhibited proliferation of CFSE-labeled target CD4+ spleen T cells more efficiently compared to CD4+CD25+ T cells isolated from the lungs of OVA-sensitized and -challenged, untreated mice. Mean values ± SEM; n = 5 mice per group; *P < 0.05. (D) Histograms of a representative cell population of CD4+ spleen cells labeled with CFSE and coincubated for 4 days with either CD4+CD25+ or CD4+CD25– cells isolated from the lungs of different groups. Percentages indicate the number of spleen CD4+/CFSE-labeled cells at day 4 (M1, 20 hours). (E) RT-PCR for the IL-6R α chain shows selective expression on Foxp3+ CD4+CD25+ lung T cells but not CD4+CD25– lung T cells. One representative experiment out of 3 is shown. (F) phospho–STAT-3 (pSTAT3) immunostaining in CD4+CD25+ cells. Spleen CD4+CD25+ T cells were incubated either with medium alone (left panel), with 20 ng/ml of IL-6 (middle panel), or with IL-6 (20 ng/ml) and anti–IL-6R antibodies (10 μg/ml) (right panel). Magnification, ×200.
Figure 8
CD4+CD25+ T cells from OVA-sensitized mice can inhibit CD4+CD25– T cell–induced experimental asthma in Rag1–/– mice. (A–F) Cotransfer of CD4+CD25+ and CD4+CD25– spleen T cells into Rag1–/– mice. CD4+CD25+ and CD4+CD25– T cells were isolated from the spleens of OVA-sensitized and -challenged mice given IgG-control antibodies or anti–IL-6R antibodies. CFSE-labeled CD4+CD25– spleen T cells from OVA-sensitized mice (5 × 105; CSFE-labeled indicated with asterisks) were cotransferred i.p. with either 5 × 105 unlabeled CD4+CD25– T cells (A and D) or CD4+CD25+ T cells (B, C, E, and F) into immunocompromised Rag1 knockout mice. Cotransfer of CD4+CD25+ T cells from anti–IL-6R–treated (C and F) or IgG-treated (B and E) mice suppressed allergic airway inflammation induced by transfer of CD4+CD25– T cells from OVA-sensitized mice. Magnification, ×100 (A–C), ×400 (D–F). Mice receiving only CD4+CD25– T cells showed CFSE-positive cells in the lung (H), whereas mice receiving CD4+CD25+ T cells as well showed a marked decrease in the number of CFSE-positive cells (G and I), suggesting a CD4+CD25+ T cell–mediated suppression of effector CD4+CD25– T cell proliferation. Results in G were obtained by calculating the average number of CFSE-positive cells per high power field (HPF) (n = 30). ***P = 0.001.
Similar articles
- Local blockade of IL-6R signaling induces lung CD4+ T cell apoptosis in a murine model of asthma via regulatory T cells.
Finotto S, Eigenbrod T, Karwot R, Boross I, Doganci A, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Rose-John S, Galle PR, Neurath MF. Finotto S, et al. Int Immunol. 2007 Jun;19(6):685-93. doi: 10.1093/intimm/dxm037. Epub 2007 May 11. Int Immunol. 2007. PMID: 17496315 - Pathological role of IL-6 in the experimental allergic bronchial asthma in mice.
Doganci A, Sauer K, Karwot R, Finotto S. Doganci A, et al. Clin Rev Allergy Immunol. 2005 Jun;28(3):257-70. doi: 10.1385/CRIAI:28:3:257. Clin Rev Allergy Immunol. 2005. PMID: 16129910 Review. - Allergen-induced IL-6 trans-signaling activates γδ T cells to promote type 2 and type 17 airway inflammation.
Ullah MA, Revez JA, Loh Z, Simpson J, Zhang V, Bain L, Varelias A, Rose-John S, Blumenthal A, Smyth MJ, Hill GR, Sukkar MB, Ferreira MA, Phipps S. Ullah MA, et al. J Allergy Clin Immunol. 2015 Oct;136(4):1065-73. doi: 10.1016/j.jaci.2015.02.032. Epub 2015 Apr 28. J Allergy Clin Immunol. 2015. PMID: 25930193 - IL-2 receptor beta-chain signaling controls immunosuppressive CD4+ T cells in the draining lymph nodes and lung during allergic airway inflammation in vivo.
Doganci A, Karwot R, Maxeiner JH, Scholtes P, Schmitt E, Neurath MF, Lehr HA, Ho IC, Finotto S. Doganci A, et al. J Immunol. 2008 Aug 1;181(3):1917-26. doi: 10.4049/jimmunol.181.3.1917. J Immunol. 2008. PMID: 18641329 - T-cell regulation in asthmatic diseases.
Finotto S. Finotto S. Chem Immunol Allergy. 2008;94:83-92. doi: 10.1159/000154869. Chem Immunol Allergy. 2008. PMID: 18802339 Review.
Cited by
- Alternaria fungus induces the production of GM-CSF, interleukin-6 and interleukin-8 and calcium signaling in human airway epithelium through protease-activated receptor 2.
Matsuwaki Y, Wada K, White T, Moriyama H, Kita H. Matsuwaki Y, et al. Int Arch Allergy Immunol. 2012;158 Suppl 1(Suppl 1):19-29. doi: 10.1159/000337756. Epub 2012 May 15. Int Arch Allergy Immunol. 2012. PMID: 22627362 Free PMC article. - Psychological factors in asthma.
Van Lieshout RJ, Macqueen G. Van Lieshout RJ, et al. Allergy Asthma Clin Immunol. 2008 Mar 15;4(1):12-28. doi: 10.1186/1710-1492-4-1-12. Epub 2008 Mar 15. Allergy Asthma Clin Immunol. 2008. PMID: 20525122 Free PMC article. - Molecular mechanisms regulating TGF-beta-induced Foxp3 expression.
Xu L, Kitani A, Strober W. Xu L, et al. Mucosal Immunol. 2010 May;3(3):230-8. doi: 10.1038/mi.2010.7. Epub 2010 Mar 10. Mucosal Immunol. 2010. PMID: 20404810 Free PMC article. Review. - Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma.
Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Jarjour NN, Gern JE. Bochkov YA, et al. Mucosal Immunol. 2010 Jan;3(1):69-80. doi: 10.1038/mi.2009.109. Epub 2009 Aug 26. Mucosal Immunol. 2010. PMID: 19710636 Free PMC article. - Unique gene expression profiles in infants vaccinated with different strains of Mycobacterium bovis bacille Calmette-Guerin.
Wu B, Huang C, Garcia L, Ponce de Leon A, Osornio JS, Bobadilla-del-Valle M, Ferreira L, Canizales S, Small P, Kato-Maeda M, Krensky AM, Clayberger C. Wu B, et al. Infect Immun. 2007 Jul;75(7):3658-64. doi: 10.1128/IAI.00244-07. Epub 2007 May 14. Infect Immun. 2007. PMID: 17502394 Free PMC article.
References
- Holgate ST. The epidemic of allergy and asthma. Nature. 1999;402:B2–B4. - PubMed
- Robinson DS, et al. Activation of CD4+ T cells, increased Th2-type mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J. Allergy Clin. Immunol. 1993;92:313–324. - PubMed
- Robinson DS, et al. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 1992;326:298–304. - PubMed
- Tang C, Rolland JM, Ward C, Quan B, Waters EH. IL-5 production by broncho-alveolar lavage and peripheral blood mononuclear cells in asthma and atopy. Eur. Respir. J. 1997;10:624–632. - PubMed
- Ying S, et al. T cells are the principal source of interleukin-5 mRNA in allergen-induced rhinitis. Am. J. Respir. Cell Mol. Biol. 1993;4:356–360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous