A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injury - PubMed (original) (raw)
. 2005 Feb 8;102(6):2046-51.
doi: 10.1073/pnas.0409329102. Epub 2005 Jan 25.
Stefano Chimenti, Lidia Staszewsky, Antonio Bai, Eleonora Carlo, Ivan Cuccovillo, Mirko Doni, Manuela Mengozzi, Rossella Tonelli, Pietro Ghezzi, Thomas Coleman, Michael Brines, Anthony Cerami, Roberto Latini
Affiliations
- PMID: 15671158
- PMCID: PMC548534
- DOI: 10.1073/pnas.0409329102
A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injury
Fabio Fiordaliso et al. Proc Natl Acad Sci U S A. 2005.
Abstract
The cytokine erythropoietin (EPO) protects the heart from ischemic injury, in part by preventing apoptosis. However, EPO administration can also raise the hemoglobin concentration, which, by increasing oxygen delivery, confounds assignment of cause and effect. The availability of EPO analogs that do not bind to the dimeric EPO receptor and lack erythropoietic activity, e.g., carbamylated EPO (CEPO), provides an opportunity to determine whether EPO possesses direct cardioprotective activity. In vivo, cardiomyocyte loss after experimental myocardial infarction (MI) of rats (40 min of occlusion with reperfusion) was reduced from approximately 57% in MI-control to approximately 45% in animals that were administered CEPO daily for 1 week (50 microg/kg of body weight s.c.) with the first dose administered intravenously 5 min before reperfusion. CEPO did not increase the hematocrit, yet it prevented increases in left ventricular (LV) end-diastolic pressure, reduced LV wall stress in systole and diastole, and improved LV response to dobutamine infusion compared with vehicle-treated animals. In agreement with the cardioprotective effect observed in vivo, staurosporine-induced apoptosis of adult rat or mouse cardiomyocytes in vitro was also significantly attenuated ( approximately 35%) by CEPO, which is comparable with the effect of EPO. These data indicate that prevention of cardiomyocyte apoptosis, in the absence of an increase in hemoglobin concentration, explains EPO's cardioprotection. Nonerythropoietic derivatives such as CEPO, devoid of the undesirable effects of EPO, e.g., thrombogenesis, could represent safer and more effective alternatives for treatment of cardiovascular diseases, such as MI and heart failure. Furthermore, these findings expand the activity spectrum of CEPO to tissues outside the nervous system.
Figures
Fig. 1.
Mean percentage of myocytes positive for TdT assay counted on 450 cells in each dish obtained by isolation from different rat or mouse hearts. Shown are data for rat control (n = 9), S (Staurosporine, n = 10), rat S plus C (S plus CEPO, n = 10), mouse S plus C (n = 4), and mouse S plus E (S plus EPO; n = 4). *, P < 0.05; **, P < 0.01 versus staurosporine.
Fig. 2.
Histomorphometric evaluation of infarct size (A), number of cardiac myocyte nuclei in left ventricle (B), cardiac myocyte volume per nucleus (C), and cross-sectional area of cardiac myocytes in the spared left ventricle of Sham-operated rats and in coronary-ligated rats untreated or treated with CEPO (D). MI-control group; CEPO, MI-CEPO group. *, P < 0.01 versus vehicle.
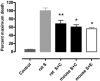
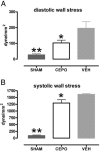
Fig. 3.
CEPO administration prevents full increase in LV wall stress in diastole (A) and in systole (B). *, P < 0.05 or **, P < 0.01 versus MI-control (VEH).
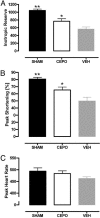
Fig. 4.
Echo dobutamine stress test for the assessment of myocardial viability in lightly sedated rats. Inotropic reserve (A), Maximum (peak) SF (B), and maximum heart rate (C) on dobutamine in Sham-operated rats and in coronary ligated rats untreated or treated with CEPO are shown. VEH, MI-control group; CEPO, MI-CEPO group. *, P < 0.05; **, P < 0.01 versus VEH group.
Similar articles
- Carbamylated erythropoietin protects the myocardium from acute ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism.
Xu X, Cao Z, Cao B, Li J, Guo L, Que L, Ha T, Chen Q, Li C, Li Y. Xu X, et al. Surgery. 2009 Sep;146(3):506-14. doi: 10.1016/j.surg.2009.03.022. Epub 2009 Jun 9. Surgery. 2009. PMID: 19715808 - Erythropoietin (EPO) affords more potent cardioprotection by activation of distinct signaling to mitochondrial kinases compared with carbamylated EPO.
Sato T, Tanno M, Miki T, Yano T, Sato T, Shimamoto K, Miura T. Sato T, et al. Cardiovasc Drugs Ther. 2010 Dec;24(5-6):401-8. doi: 10.1007/s10557-010-6265-5. Cardiovasc Drugs Ther. 2010. PMID: 20953685 - Erythropoietin, modified to not stimulate red blood cell production, retains its cardioprotective properties.
Moon C, Krawczyk M, Paik D, Coleman T, Brines M, Juhaszova M, Sollott SJ, Lakatta EG, Talan MI. Moon C, et al. J Pharmacol Exp Ther. 2006 Mar;316(3):999-1005. doi: 10.1124/jpet.105.094854. Epub 2005 Nov 23. J Pharmacol Exp Ther. 2006. PMID: 16306273 - An update on the cardiac effects of erythropoietin cardioprotection by erythropoietin and the lessons learnt from studies in neuroprotection.
Bogoyevitch MA. Bogoyevitch MA. Cardiovasc Res. 2004 Aug 1;63(2):208-16. doi: 10.1016/j.cardiores.2004.03.017. Cardiovasc Res. 2004. PMID: 15249178 Review. - Molecular basis of cardioprotection by erythropoietin.
Burger D, Xenocostas A, Feng QP. Burger D, et al. Curr Mol Pharmacol. 2009 Jan;2(1):56-69. doi: 10.2174/1874467210902010056. Curr Mol Pharmacol. 2009. PMID: 20021446 Review.
Cited by
- Longer-term outcomes of darbepoetin alfa versus epoetin alfa in patients with ESRD initiating hemodialysis: a quasi-experimental cohort study.
Winkelmayer WC, Chang TI, Mitani AA, Wilhelm-Leen ER, Ding V, Chertow GM, Brookhart MA, Goldstein BA. Winkelmayer WC, et al. Am J Kidney Dis. 2015 Jul;66(1):106-13. doi: 10.1053/j.ajkd.2015.02.339. Epub 2015 May 2. Am J Kidney Dis. 2015. PMID: 25943715 Free PMC article. - Towards comprehensive cardiac repair and regeneration after myocardial infarction: Aspects to consider and proteins to deliver.
Awada HK, Hwang MP, Wang Y. Awada HK, et al. Biomaterials. 2016 Mar;82:94-112. doi: 10.1016/j.biomaterials.2015.12.025. Epub 2015 Dec 29. Biomaterials. 2016. PMID: 26757257 Free PMC article. Review. - Suppression of coronary atherosclerosis by helix B surface Peptide, a nonerythropoietic, tissue-protective compound derived from erythropoietin.
Ueba H, Shiomi M, Brines M, Yamin M, Kobayashi T, Ako J, Momomura S, Cerami A, Kawakami M. Ueba H, et al. Mol Med. 2013 Jul 24;19(1):195-202. doi: 10.2119/molmed.2013.00037. Mol Med. 2013. PMID: 23648638 Free PMC article. - The role of PI3-K/Akt signal pathway in the antagonist effect of CEPO on CHF rats.
Huang Z, Xu W, Wu J, Chen S, Chen X. Huang Z, et al. Exp Ther Med. 2018 Dec;16(6):5161-5165. doi: 10.3892/etm.2018.6822. Epub 2018 Oct 2. Exp Ther Med. 2018. PMID: 30542471 Free PMC article. - N-Glycosylation engineering of tobacco plants to produce asialoerythropoietin.
Kittur FS, Hung CY, Darlington DE, Sane DC, Xie J. Kittur FS, et al. Plant Cell Rep. 2012 Jul;31(7):1233-43. doi: 10.1007/s00299-012-1244-x. Epub 2012 Feb 28. Plant Cell Rep. 2012. PMID: 22371257
References
- Gong, H., Wang, W., Kwon, T. H., Jonassen, T., Li, C., Ring, T., Froki, A. J. & Nielsen, S. (2004) Kidney Int. 66, 683–695. - PubMed
- Yang, C. W., Li, C., Jung, J. Y., Shin, S. J., Choi, B. S., Lim, S. W., Sun, B. K., Kim, Y. S., Kim, J., Chang, Y. S. & Bang, B. K. (2003) FASEB J. 17, 1754–1755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials