Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson's disease - PubMed (original) (raw)
Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson's disease
Andisheh Eslamboli et al. J Neurosci. 2005.
Abstract
The therapeutic potential of glial cell line-derived neurotrophic factor (GDNF) for Parkinson's disease is likely to depend on sustained delivery of the appropriate amount to the target areas. Recombinant adeno-associated viral vectors (rAAVs) expressing GDNF may be a suitable delivery system for this purpose. The aim of this study was to define a sustained level of GDNF that does not affect the function of the normal dopamine (DA) neurons but does provide anatomical and behavioral protection against an intrastriatal 6-hydroxydopamine (6-OHDA) lesion in the common marmoset. We found that unilateral intrastriatal injection of rAAV resulting in the expression of high levels of GDNF (14 ng/mg of tissue) in the striatum induced a substantial bilateral increase in tyrosine hydroxylase protein levels and activity as well as in DA turnover. Expression of low levels of GDNF (0.04 ng/mg of tissue), on the other hand, produced only minimal effects on DA synthesis and only on the injected side. In addition, the low level of GDNF provided approximately 85% protection of the nigral DA neurons and their projections to the striatum in the 6-OHDA-lesioned hemisphere. Furthermore, the anatomical protection was accompanied by a complete attenuation of sensorimotor neglect, head position bias, and amphetamine-induced rotation. We conclude that when delivered continuously, a low level of GDNF in the striatum (approximately threefold above baseline) is sufficient to provide optimal functional outcome.
Figures
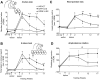
Figure 1.
Behavioral measures. A, The two-tubes task. On each of 30 trials, each monkey was presented with two tubes that were both baited with a food reward. The tubes were pseudorandomly presented to the front, left, or right of the monkey such that there were 10 trials in each position. The monkey was allowed to remove the food reward from either of the two tubes. Monkeys will usually choose the nearer, more central, tube when the pair of tubes is presented to their left or right and will choose randomly when the tubes are presented centrally. Monkeys with an ipsilesional bias differ from normal monkeys in that they choose the ipsilesional tube of the pair, regardless of the position in which they are presented. This difference is most marked when the tubes are presented on the ipsilesional side, because unlesioned monkeys will choose the nearer tube, and lesioned monkeys will choose the tube which is further away, as illustrated in A, which shows the number of pieces retrieved from the further tube. B, The six-tubes task. On each of 30 trials, each monkey was presented with a horizontal array of six tubes, one of which contained food reward. Each tube was baited five times in a pseudorandom order. The animal was allowed 30 s per trial to find the reward. Normal monkeys take very slightly longer to find a reward in an outer tube compared with the time taken to find centrally located rewards. Monkeys with unilateral 6-OHDA lesions take longer to find the reward in any tube but are particularly slow to find the reward when it is in the most contralesional tube (Milton et al., 2004). B shows the time taken to find the reward in the most contralesional tube. C, Head position bias. Each monkey was observed for 180 s/d for 3 d. C shows the average number of seconds per day that the monkeys had their heads turned ipsilesionally minus contralesionally. D, Amphetamine rotation. Each monkey was videotaped for 30 min, starting 30 min after injection with amphetamine (0.5 mg/kg, i.m.; Sigma). D shows average number of ipsilesional minus contralesional 360° rotations. After 6-OHDA lesions, there were group by week interactions (two-tubes task, F(6,45) = 6.04, p < 0.001; six-tubes task, F(6,45) = 10.50, p < 0.001; head position bias, F(8,60) = 3.676, p < 0.01). Differences at each time point were compared using Bonferroni corrected t tests. *p < 0.05 compared with INTACT; +p < 0.05, GDNF plus LES compared with GFP plus LES. # indicates that there was a group difference in amphetamine rotation (F(2,15) = 5.21; p < 0.05). The group by week interaction approached significance (F(6,45) = 2.14; p = 0.07). The “blobs” on the heads in the diagrams indicate the side of the lesion. The arrows point to the tubes for which data are shown. Error bars represent SEM.
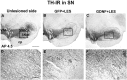
Figure 2.
TH-IR staining of the SN (AP 4.5). A, Unlesioned SN. B, Lesioned SN in a monkey from group GFP plus LES showing substantial reduction in staining. C, Lesioned SN in a monkey from group GDNF plus LES showing partial protection against the 6-OHDA lesion. _D_-F, High-power micrographs taken from _A_-C, respectively, at the locations indicted by the boxes. E shows reduction in TH-IR fibers. F shows substantial protection of the TH-IR fibers in the SN that extend into the SN pars reticulata. SNpc, SN pars compacta; SNpr, SN pars reticulata; VTA, ventral tegmental area; cp, cerebral peduncles. Scale bars: (in A) _A_-C, 1 mm; (in D) _D_-F, 0.1 mm.
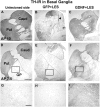
Figure 3.
TH-IR staining of the basal ganglia (AP10.0 and 7.5). A, D, Unlesioned basal ganglia. B, E, Lesioned basal ganglia in a monkey from group GFP plus LES showing substantial but subtotal reduction in staining in caudate (Caud) and putamen (Put). C, F, Lesioned basal ganglia in a monkey from group GDNF plus LES showing partial protection against the 6-OHDA lesion. The caudate, putamen, and globus pallidus (GP) are delineated by lines. _G_-I, High-power micrographs taken from _D_-F, respectively, at the locations indicated by the boxes. The arrowhead in B shows needle track. Scale bars: (in A, D) _A_-F, 1 mm; (in G) _G_-I, 0.1 mm. VMAT2-IR pictures are not shown but look similar to the TH-IR figures.
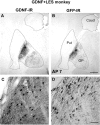
Figure 4.
GDNF-IR (A, C) and GFP-IR (B, D) reactivity in basal ganglia in a monkey from group GDNF plus LES. Scale bars: (in B) A, B, 1 mm; (in C) C, D, 50 μm. Asterisks in A and B refer to locations of panels C and D, respectively. Caud, Caudate; Put, putamen; GP, globus pallidus.
Similar articles
- Recombinant adeno-associated viral vector (rAAV) delivery of GDNF provides protection against 6-OHDA lesion in the common marmoset monkey (Callithrix jacchus).
Eslamboli A, Cummings RM, Ridley RM, Baker HF, Muzyczka N, Burger C, Mandel RJ, Kirik D, Annett LE. Eslamboli A, et al. Exp Neurol. 2003 Nov;184(1):536-48. doi: 10.1016/j.expneurol.2003.08.007. Exp Neurol. 2003. PMID: 14637123 - Delivery of a GDNF gene into the substantia nigra after a progressive 6-OHDA lesion maintains functional nigrostriatal connections.
Kozlowski DA, Connor B, Tillerson JL, Schallert T, Bohn MC. Kozlowski DA, et al. Exp Neurol. 2000 Nov;166(1):1-15. doi: 10.1006/exnr.2000.7463. Exp Neurol. 2000. PMID: 11031079 - Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model.
Björklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel RJ. Björklund A, et al. Brain Res. 2000 Dec 15;886(1-2):82-98. doi: 10.1016/s0006-8993(00)02915-2. Brain Res. 2000. PMID: 11119690 Review. - Adenoviral vector-mediated delivery of glial cell line-derived neurotrophic factor provides neuroprotection in the aged parkinsonian rat.
Connor B. Connor B. Clin Exp Pharmacol Physiol. 2001 Nov;28(11):896-900. doi: 10.1046/j.1440-1681.2001.03544.x. Clin Exp Pharmacol Physiol. 2001. PMID: 11703392 Review.
Cited by
- Clinically relevant effects of convection-enhanced delivery of AAV2-GDNF on the dopaminergic nigrostriatal pathway in aged rhesus monkeys.
Johnston LC, Eberling J, Pivirotto P, Hadaczek P, Federoff HJ, Forsayeth J, Bankiewicz KS. Johnston LC, et al. Hum Gene Ther. 2009 May;20(5):497-510. doi: 10.1089/hum.2008.137. Hum Gene Ther. 2009. PMID: 19203243 Free PMC article. - The cell biology of Parkinson's disease.
Panicker N, Ge P, Dawson VL, Dawson TM. Panicker N, et al. J Cell Biol. 2021 Apr 5;220(4):e202012095. doi: 10.1083/jcb.202012095. J Cell Biol. 2021. PMID: 33749710 Free PMC article. Review. - Autologous transplantation of GDNF-expressing mesenchymal stem cells protects against MPTP-induced damage in cynomolgus monkeys.
Ren Z, Wang J, Wang S, Zou C, Li X, Guan Y, Chen Z, Zhang YA. Ren Z, et al. Sci Rep. 2013 Sep 27;3:2786. doi: 10.1038/srep02786. Sci Rep. 2013. PMID: 24071770 Free PMC article. - Neurotrophins as Therapeutic Agents for Parkinson's Disease; New Chances From Focused Ultrasound?
Stefani A, Pierantozzi M, Cardarelli S, Stefani L, Cerroni R, Conti M, Garasto E, Mercuri NB, Marini C, Sucapane P. Stefani A, et al. Front Neurosci. 2022 Mar 25;16:846681. doi: 10.3389/fnins.2022.846681. eCollection 2022. Front Neurosci. 2022. PMID: 35401084 Free PMC article. Review. - Biosynthesis, processing, and secretion of glial cell line-derived neurotrophic factor in astroglial cells.
Oh-hashi K, Ito M, Tanaka T, Hirata Y, Kiuchi K. Oh-hashi K, et al. Mol Cell Biochem. 2009 Mar;323(1-2):1-7. doi: 10.1007/s11010-008-9958-3. Epub 2008 Nov 13. Mol Cell Biochem. 2009. PMID: 19005738
References
- Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, Rosenthal A, Hefti F (1995) Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature 373: 339-341. - PubMed
- Bjorklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel RJ (2000) Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res 886: 82-98. - PubMed
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N (2004) Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther 10: 302-317. - PubMed
- Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC (1997) Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science 275: 838-841. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical