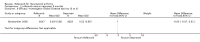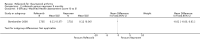Rofecoxib for rheumatoid arthritis - PubMed (original) (raw)
Review
Rofecoxib for rheumatoid arthritis
S E Garner et al. Cochrane Database Syst Rev. 2005.
Abstract
Background: Editor's note: The anti-inflammatory drug rofecoxib (Vioxx) was withdrawn from the market at the end of September 2004 after it was shown that long-term use (greater than 18 months) could increase the risk of heart attack and stroke. Further information is available at www.vioxx.com. Rheumatoid arthritis (RA) is a systemic auto-immune disorder, in which the synovial lining of many joints and tendon sheaths are persistently inflamed.
Objectives: To assess the efficacy and toxicity of rofecoxib for treating RA.
Search strategy: We searched the following electronic databases up to December 2000: MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Controlled Trials Register, National Research Register, NHS Economic Evaluation Database, Health Technology Assessment database. The bibliographies of retrieved papers were scanned for additional references. The manufacturers of rofecoxib, MSD, were also approached by the UK National Institute for Clinical Excellence to submit additional evidence to inform it's appraisal on the use of cyclo-oxygenase inhibitors for arthritis.
Selection criteria: We included randomised controlled trials of parallel group design evaluating the efficacy and/or toxicity of rofecoxib in RA, both placebo based and comparative trials were eligible. Relevant outcome criteria had to be available to evaluate efficacy and/or toxicity, such as the OMERACT outcomes.
Data collection and analysis: Data were abstracted independently by two reviewers and the results were compared for the degree of agreement. A validated tool (Jadad 1996) was used to score the quality of the randomised controlled trials. The planned analysis was to pool, where appropriate, continuous outcome measures using mean or standardized mean differences, and dichotomous outcome measures using relative risk ratios.
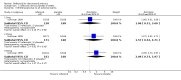
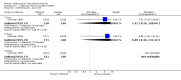
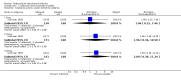
Main results: Two randomised controlled trials evaluating rofecoxib for the treatment of RA were identified and met the inclusion criteria. One compared rofecoxib to placebo and was designed to assess the safety and efficacy of several doses of rofecoxib. The second trial compared rofecoxib to naproxen and was primarily designed to assess the safety of rofecoxib so did not include all the recommended RA efficacy measures. The overall number of ACR 20 responders who had received 25mg (82/ 171 = 48%) or 50mg (86/161 = 53%) was statistically significantly more than those receiving placebo (58/168 = 35% ) (RR 1.39 CI: 1.07, 1.80 and RR 1.55 CI: 1.20, 1.99 respectively) with no statistically significant differences between the 25 and 50 mg doses. The safety profile of rofecoxib was similar to that of placebo. In the comparative trial, rofecoxib at a dosage of 50 mg/day demonstrated similar efficacy to naproxen at a dosage of 500 mg twice daily. However, the combined rate of clinically significant complicated gastro-intestinal events (GI) (perforations, ulcers, bleeds, or obstructions) was lower with rofecoxib than with naproxen (RR 0.46, 95% CI, 0.34 to 0.63) due to a reduction in the number of ulcers and bleeds. Compared to patients taking naproxen, patients taking rofecoxib had a greater risk of having any cardiovascular event (45/4047 = 1.1% vs 19/4029 =0.47%) (RR 2.36 CI 1.38 to 4.02) and had greater risk of having a non-fatal myocardial infarction (MI) (18/4047 =0 .44% and 4/4029 =0.1%) (RR 4.48, 95% CI, 1.52 to 13.23).
Authors' conclusions: In patients with RA, rofecoxib demonstrates a greater degree of efficacy than placebo, while having a comparable safety profile. Rofecoxib demonstrates a similar degree of efficacy as naproxen, but with a significantly lower rate of ulceration and gastrointestinal bleeding. Rofecoxib was associated with a greater risk for MI, but the exact significance and pathophysiology of this possible relationship is unclear. Rofecoxib was voluntarily withdrawn from global markets in October 2004. It cannot therefore be prescribed and therefore there are no implications for practice concerning its use. None the less when considering which NSAID to use, it must be borne in mind that the toxicity of NSAIDs is variable amongst patients and drugs and it tends to be dose related and associated with variation in the mode of action, absorption, distribution and metabolism. There remains a number of questions over both the benefits and risks associated with Cox II selective agents and further work is ongoing. It is likely that this issue will not be resolved until research has enabled a fuller understanding of the complex mechanism by which the Cox system operates.
Conflict of interest statement
None known
Figures
1.1. Analysis
Comparison 1 rofecoxib versus placebo 8 weeks, Outcome 1 Withdrawals ‐all.
1.2. Analysis
Comparison 1 rofecoxib versus placebo 8 weeks, Outcome 2 Withdrawals‐due to lack of efficacy.
1.3. Analysis
Comparison 1 rofecoxib versus placebo 8 weeks, Outcome 3 Withdrawals‐due to all adverse events.
1.4. Analysis
Comparison 1 rofecoxib versus placebo 8 weeks, Outcome 4 Withdrawals ‐ due to GI.
1.5. Analysis
Comparison 1 rofecoxib versus placebo 8 weeks, Outcome 5 Adverse events‐Hypertension.
1.6. Analysis
Comparison 1 rofecoxib versus placebo 8 weeks, Outcome 6 Adverse events ‐Lower extremity oedema.
1.7. Analysis
Comparison 1 rofecoxib versus placebo 8 weeks, Outcome 7 Efficacy ‐ACR 20 responders.
1.8. Analysis
Comparison 1 rofecoxib versus placebo 8 weeks, Outcome 8 Efficacy ‐ACR 20 responders: methotrexate users.
1.9. Analysis
Comparison 1 rofecoxib versus placebo 8 weeks, Outcome 9 Efficacy ‐ACR 20 responders: methotrexate nonusers.
2.1. Analysis
Comparison 2 rofecoxib versus naproxen 9 months, Outcome 1 Withdrawals ‐all.
2.2. Analysis
Comparison 2 rofecoxib versus naproxen 9 months, Outcome 2 Withdrawals ‐due to lack of efficacy.
2.3. Analysis
Comparison 2 rofecoxib versus naproxen 9 months, Outcome 3 Withdrawals ‐due to adverse events.
2.4. Analysis
Comparison 2 rofecoxib versus naproxen 9 months, Outcome 4 Adverse events ‐Incidence of GI events.
2.5. Analysis
Comparison 2 rofecoxib versus naproxen 9 months, Outcome 5 Adverse events‐ Cardiovascular Thrombotic.
2.6. Analysis
Comparison 2 rofecoxib versus naproxen 9 months, Outcome 6 Adverse events ‐Renal function.
2.7. Analysis
Comparison 2 rofecoxib versus naproxen 9 months, Outcome 7 Efficacy ‐Patient Global Disease Activity (0 to 4).
2.8. Analysis
Comparison 2 rofecoxib versus naproxen 9 months, Outcome 8 Efficacy ‐Investigator Global Disease Activity (0 to 4).
2.9. Analysis
Comparison 2 rofecoxib versus naproxen 9 months, Outcome 9 Efficacy ‐Modified Health Assessment Score (0 to 3).
Update of
- Rofecoxib for the treatment of rheumatoid arthritis.
Garner S, Fidan D, Frankish R, Judd M, Towheed T, Wells G, Tugwell P. Garner S, et al. Cochrane Database Syst Rev. 2002;(3):CD003685. doi: 10.1002/14651858.CD003685. Cochrane Database Syst Rev. 2002. PMID: 12137705 Updated. Review.
Similar articles
- Rofecoxib for the treatment of rheumatoid arthritis.
Garner S, Fidan D, Frankish R, Judd M, Towheed T, Wells G, Tugwell P. Garner S, et al. Cochrane Database Syst Rev. 2002;(3):CD003685. doi: 10.1002/14651858.CD003685. Cochrane Database Syst Rev. 2002. PMID: 12137705 Updated. Review. - Rofecoxib for the treatment of rheumatoid arthritis.
Garner S, Fidan D, Frankish R, Judd M, Towheed T, Wells G, Tugwell P. Garner S, et al. Cochrane Database Syst Rev. 2002;(2):CD003685. doi: 10.1002/14651858.CD003685. Cochrane Database Syst Rev. 2002. PMID: 12076502 Updated. Review. - Rofecoxib for osteoarthritis.
Garner SE, Fidan DD, Frankish R, Maxwell L. Garner SE, et al. Cochrane Database Syst Rev. 2005 Jan 25;2005(1):CD005115. doi: 10.1002/14651858.CD005115. Cochrane Database Syst Rev. 2005. PMID: 15654705 Free PMC article. Review. - Non-steroidal anti-inflammatory drugs (NSAIDs) for chronic non-cancer pain in children and adolescents.
Eccleston C, Cooper TE, Fisher E, Anderson B, Wilkinson NM. Eccleston C, et al. Cochrane Database Syst Rev. 2017 Aug 2;8(8):CD012537. doi: 10.1002/14651858.CD012537.pub2. Cochrane Database Syst Rev. 2017. PMID: 28770976 Free PMC article. Review. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
- Non-steroidal anti-inflammatory drugs for acute gout.
van Durme CM, Wechalekar MD, Landewé RB, Pardo Pardo J, Cyril S, van der Heijde D, Buchbinder R. van Durme CM, et al. Cochrane Database Syst Rev. 2021 Dec 9;12(12):CD010120. doi: 10.1002/14651858.CD010120.pub3. Cochrane Database Syst Rev. 2021. PMID: 34882311 Free PMC article. Review. - Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases.
Gabriel SE, Michaud K. Gabriel SE, et al. Arthritis Res Ther. 2009;11(3):229. doi: 10.1186/ar2669. Epub 2009 May 19. Arthritis Res Ther. 2009. PMID: 19519924 Free PMC article. Review. - Comparison of cardiorenal safety of nonsteroidal anti-inflammatory drugs in the treatment of arthritis: a network meta-analysis.
Wang K, Li X. Wang K, et al. Ann Transl Med. 2022 Dec;10(24):1388. doi: 10.21037/atm-22-6181. Ann Transl Med. 2022. PMID: 36660678 Free PMC article. - The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis.
Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, Siu S, Kraft J, Lynde C, Pope J, Gulliver W, Keeling S, Dutz J, Bessette L, Bissonnette R, Haraoui B. Roubille C, et al. Ann Rheum Dis. 2015 Mar;74(3):480-9. doi: 10.1136/annrheumdis-2014-206624. Epub 2015 Jan 5. Ann Rheum Dis. 2015. PMID: 25561362 Free PMC article. Review. - Prevention of stroke in rheumatoid arthritis.
Dhillon N, Liang K. Dhillon N, et al. Curr Treat Options Neurol. 2015 Jul;17(7):356. doi: 10.1007/s11940-015-0356-3. Curr Treat Options Neurol. 2015. PMID: 25981388
References
References to studies included in this review
Bombardier 2000 {published data only}
- Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of Rofecoxib and Naproxen in patients with rheumatoid arthritis. The VIGOR trial. New England Journal of Medicine 2000;343(21):1520‐1584. - PubMed
Schnitzer 1999 {published data only}
- Schnitzer TJ, Truitt K, Fleischmann R, Dalgin P, Block J, Zeng Q, et al. The safety profile, tolerability, and effective dose range of rofecoxib in the treatment of rheumatoid arthritis. Phase II Rofecoxib Rheumatoid Arthritis Study Group. Clinical Therapeutics 1999;21(10):1688‐1702. - PubMed
References to studies excluded from this review
Bjarnason 1998 {published data only}
- Bjarnason I SGCReal. COX‐2 specific inhibition with MK‐096625 or 50 mg Q.D. does not increase intestinal permeability [abstract]. American journal of Gastroenterology 1998;93(9):1670‐Abstract 246.
Lanza 1999 {published data only}
- Lanza FL, Rack MF, Simon TJ, Quan H, Bolognese JA, Hoover ME, et al. Specific inhibition of cyclooxygenase‐2 with MK‐0966 is associated with less gastroduodenal damage than either aspirin or ibuprofen. Alimentary Pharmacology & Therapeutics 1999;13(6):761‐767. - PubMed
Malmstrom 1999 {published data only}
- Malmstrom K, Daniels S, Kotey P, Seidenberg B, Desjardins P. Comparison of rofecoxib and celecoxib, two cyclooxygenase‐2 inhibitors in postoperative dental pain: a randomized, placebo‐ and active‐comparator‐controlled clinical trial. Clinical Therapeutics 1999;21(10):1653‐1663. - PubMed
Sigthrosson 2000 {published data only}
References to studies awaiting assessment
CDER 017 {unpublished data only}
- 6 week placebo controlled study 137 patients (125mg rofecoxib, 175mg rofecoxib versus placebo). Centre for Drug Evaluation and Research (CDER017) plus extension.
Hawkey 2003 {published data only}
Study 1 {unpublished data only}
- Phase IIb dose finding study. 2 year duration, 634 patients comparing 25, 50 mg rofecoxib to naproxen, placebo. Details in Konstam 2001.
Study 2 {unpublished data only}
- Phase III: domestic US 1 year duration, 909 patients. Comparing 12.5, 25, 50mg rofecoxib with naproxen, placebo. Details in Konstam 2001.
Truitt (FRI0037) {unpublished data only}
- Phase III international study. 1058 enrolled, 1 year duration. Rofecoxib 25, 50mg compared to naproxen, placebo. Details in Konstam 2001.
References to ongoing studies
Moots 2001 {unpublished data only}
- Measurement of clinical outcome within clinical service. Ongoing study 01/06/1999.
Additional references
Brater 2001
- Brater DC, Harris C, Redfern JS, Gertz BJ. Renal effects of COX‐2‐selective inhibitors. American Journal of Nephrology 2001;21(1):1‐15. - PubMed
Collins 1999
- Collins R, Peto R, MacMahon S, et al. Blood presure, stroke and coronary disease, II: short‐term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335:827‐838. - PubMed
Daniels 1999
- Daniels B, Seidenberg B. Cardiovascular safety profile of rofecoxib in controlled clinical trials. Arthritis & Rheumatism 1999;42(Suppl 9):S143‐Abstract 435.
FDA 2001
- FDA Advisory Committee. Cardiovascular safety review of rofecoxib. http://www.fda.gov/ohrms/dockets/ac/01/briefing/3677b2_06_cardio.pdf.
Felson 1993
- Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis and Rheumatism 1993;36:729‐40. - PubMed
Felson 1995
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith D, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis and Rheumatism 1995;38:727‐35. - PubMed
Jadad 1996
- Jadad AR, Moore RA, Carrol D, Jenkinson C. Assessing the quality of reports of randomised controlled trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Konstam 2001
- Konstam M, Weir R, Reicin A, Shapiro D, et al. Cardiovascular thrombotic events in controlled clinical trials of rofecoxib. Circulation 2001;104:r15‐r23. - PubMed
OMERACT 1993
- OMERACT. Conference on Outcome Measures in Rheumatoid Arthritis Clinical Trials. J Rheumatol 1993;20:526‐91. - PubMed
Pincus 1999
- Pincus T, Stein CM. ACR20: clinical or statistical significance?. Arthritis Rheum 1999;42(8):1572‐6. - PubMed
Rocha 2001
- Rocha JL, Fernandez‐Alonso. Acute tubulointerstitial nephritis associated wiht the selective COX II enzyme inhibitor rofecoxib. The Lancet 2001;357:9272. - PubMed
Schultz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman D. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273:408‐12. - PubMed
Silman 1998
- Silman A. Epidemiology and rheumatic diseases. In: Maddison P, Isenberg D, Woo P, Glass D editor(s). Oxford Textbook of Rheumatology. Oxford: Oxford University Press, 1998:811‐28.
Silverstein 1995
- Siverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving NSAIDs: a randomised double‐blind, placebo controlled trial. Annals of Internal Medicine 1995;123:241‐49. - PubMed
Silverstein 2000
- Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long‐term Arthritis Safety Study. JAMA 2000;284(10):1247‐55. - PubMed
Swan 2000
- Swan SK, Rudy DW, Lasseter KC, Ryan CF, et al. Effect of cyclooxygenase ‐2 inhibition on renal function in elderly persons receiving a low salt diet: a randomised controlled trial. Annals of Internal Medicine 2000;133(1):1‐9. - PubMed
Vane 1998
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annual Review of Pharmacology & Toxicology 1998;38:97‐120. - PubMed
Wallberg 1999
- Wallberg‐Jonsson S, Johansson H, Ohman ML. Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis: a retrospective cohort study from disease onset. Journal of Rheumatology 1999;26:2562‐2571. - PubMed
Watson 2000
- Watson M, Brooks S, Kirwan J, Faulkner A. Non‐aspirin, non‐steroidal anti‐inflammatory drugs for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2000, Issue 2. - PubMed
Whelton 2001
- Whelton A, Fort JG, Puma JA, et al. Cyclo‐oxygenase‐2 specific inhibitors and cardio‐renal function: a randomised controlled trial of celecoxib and rofecoxib in older hypertensive osteoarthritis patients. American Journal of Therapeutics 2001;8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous