Roles of the ITAM and PY motifs of Epstein-Barr virus latent membrane protein 2A in the inhibition of epithelial cell differentiation and activation of {beta}-catenin signaling - PubMed (original) (raw)
Roles of the ITAM and PY motifs of Epstein-Barr virus latent membrane protein 2A in the inhibition of epithelial cell differentiation and activation of {beta}-catenin signaling
Jennifer A Morrison et al. J Virol. 2005 Feb.
Abstract
Epstein-Barr virus (EBV) latent membrane protein 2A (LMP2A) is important for maintenance of latency in infected B lymphocytes. Through its immunoreceptor tyrosine-based activation motif (ITAM) and PY motifs, LMP2A is able to block B-cell receptor (BCR) signaling, bind BCR-associated kinases, and manipulate the turnover of itself and these kinases via a PY-mediated interaction with the Nedd4 family of ubiquitin ligases. In epithelial cells, LMP2A has been shown to activate the phosphatidylinositol 3'-OH kinase/Akt and beta-catenin signaling pathways. In the present study, the biological consequences of LMP2A expression in the normal human foreskin keratinocyte (HFK) cell line were investigated and the importance of the ITAM and PY motifs for LMP2A signaling effects in HFK cells was ascertained. The ITAM was essential for the activation of Akt by LMP2A in HFK cells, while both the ITAM and PY motifs contributed to LMP2A-mediated accumulation and nuclear translocation of the oncoprotein beta-catenin. LMP2A inhibited induction of differentiation in an assay conducted with semisolid methylcellulose medium, and the PY motifs were critical for this inhibition. LMP2A is expressed in the EBV-associated epithelial malignancies nasopharyngeal carcinoma and gastric carcinoma, and these data indicate that LMP2A affects cellular processes that likely contribute to carcinogenesis.
Figures
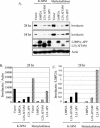
FIG. 1.
The PY motif of LMP2A is important for inhibition of involucrin expression. (A) HFK cells (4 × 106) stably expressing the pBabe vector, LMP2A, LMP2A ΔITAM, and LMP2A ΔPY were resuspended in K-SFM-methylcellulose medium. An equal number of cells were plated in parallel in tissue culture plates with K-SFM. Cells were harvested after 28 and 54 h, and immunoblot analyses were performed. Induction of differentiation was assessed with antibodies against involucrin, and expression of the LMP2A constructs was determined via anti-HA immunoblotting. Antiactin was included as a loading control. (B and C) Densitometry was performed, and involucrin levels at 28 h were normalized to the corresponding actin levels (B) or to expression of LMP2A (C) and graphed.
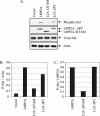
FIG. 2.
The ITAM is required for LMP2A-mediated Akt activation. (A) HFK cells stably expressing the pBabe vector, LMP2A, LMP2A ΔITAM, and LMP2A ΔPY were harvested at confluency after 48 h of starvation (i.e., with BPE and EGF supplements withheld). Activated Akt was detected by immunoblotting for activated phospho-Akt (P-Akt) in comparison with levels of total Akt. Expression levels of wild-type and mutant LMP2A were determined via anti-HA immunoblotting. Antiactin was included to control for protein loading. (B and C) Densitometry was performed, and phospho-Akt levels were normalized to the corresponding actin levels (B) or to expression of LMP2A (C) and graphed.
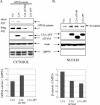
FIG. 3.
The ITAM and PY motifs contribute to the effects of LMP2A on β-catenin stabilization and nuclear translocation. HFK cells were cotransfected with GFP-β-catenin in combination with pSG5, pSG5-LMP2A, pSG5-LMP2A ΔITAM, or pSG5-LMP2A ΔPY. Supplements were withheld for 24 h prior to harvesting. Upon harvesting, cells were fractionated into their cytosolic (A) and nuclear (B) compartments and analyzed by immunoblotting. (A, top) Anti-GFP immunoblotting determined levels of GFP-β-catenin in the cytosols of transfected cells, and anti-HA detected expression of the LMP2A constructs. Antiactin and anti-GRP78 blot assays were included to assess protein loading and the integrity of the fractionation procedure, respectively. (A, bottom) Densitometry was performed, and GFP-β-catenin levels were normalized to the expression of LMP2A and graphed. (B, top) Nuclear β-catenin was detected with antibodies against β-catenin, and LMP2A expression was determined with anti-HA antibodies. GRP78 is an endoplasmic reticulum marker, and anti-GRP78 immunoblotting indicated the purity of the nuclear extracts. An antiactin immunoblot served as a protein loading control. (B, bottom) Densitometry was performed, and β-catenin levels were normalized to the expression of LMP2A and graphed. Vec, vector; exp, exposure.
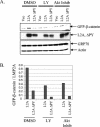
FIG. 4.
PI3K and Akt activation regulates basal levels of β-catenin in LMP2A-expressing cells. (A) HFK cells were transfected with pSG5, pSG5-LMP2A, and pSG5-LMP2A ΔPY. EGF and BPE supplements were withheld for 48 h, and the cells were treated with 25 μM LY, 10 μM Akt inhibitor, or a DMSO control for 24 h prior to being harvested. At 48 h after transfection, cells were harvested, fractionated to isolate the cytosol, and subjected to immunoblot analysis to determine cytosolic β-catenin levels (top). Anti-HA immunoblotting revealed levels of the transfected LMP2A constructs (middle), and antiactin and anti-GRP78 (an endoplasmic reticulum chaperone) were included as loading controls (bottom). (B) Densitometry was performed, and GFP-β-catenin levels were normalized to the expression of LMP2A and graphed. Vec, vector; Inhib, inhibitor.
FIG. 5.
Constitutively active PI3K, but not myr-Akt, stabilizes cytoplasmic β-catenin. HFK cells were cotransfected with GFP-β-catenin in combination with vector (Vec), LMP2A, myr-Akt, PI3K CAAX, and PI3K K227E. Supplements were withheld, and cells were treated with DMSO or 25 μM LY for 24 h prior to being harvested. Cells were analyzed by immunoblotting with anti-GFP antibodies to detect levels of GFP-β-catenin in the cytosol. Anti-GRP78 immunoblotting revealed equal loading of cytosolic proteins.
Similar articles
- Epstein-Barr virus latent membrane protein 2A activates beta-catenin signaling in epithelial cells.
Morrison JA, Klingelhutz AJ, Raab-Traub N. Morrison JA, et al. J Virol. 2003 Nov;77(22):12276-84. doi: 10.1128/jvi.77.22.12276-12284.2003. J Virol. 2003. PMID: 14581564 Free PMC article. - PY motifs of Epstein-Barr virus LMP2A regulate protein stability and phosphorylation of LMP2A-associated proteins.
Ikeda M, Ikeda A, Longnecker R. Ikeda M, et al. J Virol. 2001 Jun;75(12):5711-8. doi: 10.1128/JVI.75.12.5711-5718.2001. J Virol. 2001. PMID: 11356981 Free PMC article. - Epstein-Barr virus latent membrane protein-2A induces ITAM/Syk- and Akt-dependent epithelial migration through αv-integrin membrane translocation.
Fotheringham JA, Coalson NE, Raab-Traub N. Fotheringham JA, et al. J Virol. 2012 Oct;86(19):10308-20. doi: 10.1128/JVI.00853-12. Epub 2012 Jul 25. J Virol. 2012. PMID: 22837212 Free PMC article. - Latent Membrane Protein 2 (LMP2).
Cen O, Longnecker R. Cen O, et al. Curr Top Microbiol Immunol. 2015;391:151-80. doi: 10.1007/978-3-319-22834-1_5. Curr Top Microbiol Immunol. 2015. PMID: 26428374 Review. - The signaling pathways of Epstein-Barr virus-encoded latent membrane protein 2A (LMP2A) in latency and cancer.
Pang MF, Lin KW, Peh SC. Pang MF, et al. Cell Mol Biol Lett. 2009;14(2):222-47. doi: 10.2478/s11658-008-0045-2. Epub 2008 Dec 13. Cell Mol Biol Lett. 2009. PMID: 19082921 Free PMC article. Review.
Cited by
- Nasopharyngeal carcinoma signaling pathway: an update on molecular biomarkers.
Tulalamba W, Janvilisri T. Tulalamba W, et al. Int J Cell Biol. 2012;2012:594681. doi: 10.1155/2012/594681. Epub 2012 Mar 5. Int J Cell Biol. 2012. PMID: 22500174 Free PMC article. - Epstein-Barr virus latent membrane protein 2A contributes to anoikis resistance through ERK activation.
Iwakiri D, Minamitani T, Samanta M. Iwakiri D, et al. J Virol. 2013 Jul;87(14):8227-34. doi: 10.1128/JVI.01089-13. Epub 2013 May 22. J Virol. 2013. PMID: 23698301 Free PMC article. - Epstein-Barr virus latent membrane protein-2A-induced DeltaNp63alpha expression is associated with impaired epithelial-cell differentiation.
Fotheringham JA, Mazzucca S, Raab-Traub N. Fotheringham JA, et al. Oncogene. 2010 Jul 29;29(30):4287-96. doi: 10.1038/onc.2010.175. Epub 2010 May 24. Oncogene. 2010. PMID: 20498633 Free PMC article. - Screening Key Genes and Biological Pathways in Nasopharyngeal Carcinoma by Integrated Bioinformatics Analysis.
Tai J, Park J, Han M, Kim TH. Tai J, et al. Int J Mol Sci. 2022 Dec 11;23(24):15701. doi: 10.3390/ijms232415701. Int J Mol Sci. 2022. PMID: 36555343 Free PMC article. - Epstein-Barr virus associated modulation of Wnt pathway is not dependent on latent membrane protein-1.
Webb N, Connolly G, Tellam J, Yap AS, Khanna R. Webb N, et al. PLoS One. 2008 Sep 22;3(9):e3254. doi: 10.1371/journal.pone.0003254. PLoS One. 2008. PMID: 18806872 Free PMC article.
References
- Boukamp, P., S. Popp, S. Altmeyer, A. Hulsen, C. Fasching, T. Cremer, and N. E. Fusenig. 1997. Sustained nontumorigenic phenotype correlates with a largely stable chromosome content during long-term culture of the human keratinocyte line HaCaT. Genes Chromosomes Cancer 19:201-214. - PubMed
- Brazil, D. P., and B. A. Hemmings. 2001. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 26:657-664. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous