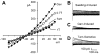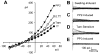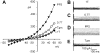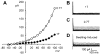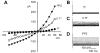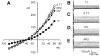Antagonistic regulation of swelling-activated Cl- current in rabbit ventricle by Src and EGFR protein tyrosine kinases - PubMed (original) (raw)
Antagonistic regulation of swelling-activated Cl- current in rabbit ventricle by Src and EGFR protein tyrosine kinases
Zuojun Ren et al. Am J Physiol Heart Circ Physiol. 2005 Jun.
Abstract
Regulation of swelling-activated Cl(-) current (I(Cl,swell)) is complex, and multiple signaling cascades are implicated. To determine whether protein tyrosine kinase (PTK) modulates I(Cl,swell) and to identify the PTK involved, we studied the effects of a broad-spectrum PTK inhibitor (genistein), selective inhibitors of Src (PP2, a pyrazolopyrimidine) and epidermal growth factor receptor (EGFR) kinase (PD-153035), and a protein tyrosine phosphatase (PTP) inhibitor (orthovanadate). I(Cl,swell) evoked by hyposmotic swelling was increased 181 +/- 17% by 100 microM genistein, and the genistein-induced current was blocked by the selective I(Cl,swell) blocker tamoxifen (10 microM). Block of Src with PP2 (10 microM) stimulated tamoxifen-sensitive I(Cl,swell) by 234 +/- 27%, mimicking genistein, whereas the inactive analog of PP2, PP3 (10 microM), had no effect. Moreover, block of PTP by orthovanadate (1 mM) inhibited I(Cl,swell) and prevented its stimulation by PP2. In contrast with block of Src, block of EGFR kinase with PD-153035 (20 nM) inhibited I(Cl,swell). Several lines of evidence argue that the PP2-stimulated current was I(Cl,swell): 1) the stimulation was volume dependent, 2) the current was blocked by tamoxifen, 3) the current outwardly rectified with both symmetrical and physiological Cl(-) gradients, and 4) the current reversed near the Cl(-) equilibrium potential. To rule out contributions of other currents, Cd(2+) (0.2 mM) and Ba(2+) (1 mM) were added to the bath. Surprisingly, Cd(2+) suppressed the decay of I(Cl,swell), and Cd(2+) plus Ba(2+) eliminated time-dependent currents between -100 and +100 mV. Nevertheless, these divalent ions did not eliminate I(Cl,swell) or prevent its stimulation by PP2. The results indicate that tyrosine phosphorylation controls I(Cl,swell), and regulation of I(Cl,swell) by the Src and EGFR kinase families of PTK is antagonistic.
Figures
Fig. 1
Genistein, a broad-spectrum protein tyrosine kinase (PTK) blocker, augmented swelling-activated Cl− current (_I_Cl,swell). A: current-voltage (I-V) relationship in isosmotic (1T) solution, after swelling in hypoosmotic (0.7T) solution for 10 min, after exposure to 100 μM genistein in 0.7T solution for 10 min (Gen), and after addition of 10 μM tamoxifen (Tam), a selective _I_Cl,swell blocker, to 0.7T plus genistein solution. I-V curves cross near the Cl− equilibrium potential (_E_Cl), which is −42 mV. B: families of swelling-induced difference currents. _I_Cl,swell partially inactivated at positive potentials. C: genistein-induced difference currents in 0.7T solution. D: tamoxifen-sensitive difference currents in 0.7T plus genistein solution. Swelling activated outwardly rectifying _I_Cl,swell that reversed near _E_Cl and was stimulated by genistein. Tamoxifen blocked both the swelling- and genistein-induced components. Calibrations apply to all current records.
Fig. 2
_I_Cl,swell is stimulated by PP2, a selective inhibitor of Src family PTKs, but not by its inactive analog, PP3. A: I-V relationships in 1T solution, after swelling in 0.7T solution for 10 min, after exposure to 10 μM PP2 in 0.7T solution for 10 min (PP2), and after addition of 10 μM tamoxifen to 0.7T solution plus PP2. B: families of swelling-induced difference currents. C: PP2-induced difference currents in 0.7T solution. D: tamoxifen-sensitive difference currents in 0.7T plus PP2 solution. Both the PP2- and swelling-induced currents were sensitive to 10 μM tamoxifen. Selective inhibition of Src by PP2 mimicked the effect of genistein. In contrast, PP3 (an inactive analog of PP2; 10 μM for 10 min) did not alter the magnitude or time course of membrane currents in 0.7T solution. E: PP3-induced difference currents in 0.7T solution. These records were obtained in a different cell than for _A_-D.
Fig. 3
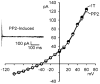
Stimulation of _I_Cl,swell by PP2 requires cell swelling. I-V relationships in 1T and after exposure to 10 μM PP2 in 1T solution for 10 min are shown. Inset: families of PP2-induced difference currents in 1T solution. PP2 did not alter the outwardly rectifying background Cl− current attributed at least in part to _I_Cl,swell under isosmotic conditions. Note the change in scale of I-V relationship compared with previous figures.
Fig. 4
Cd2+ (0.2 mM) suppressed time dependence of _I_Cl,swell at positive potentials and revealed a delayed rectifier current. Examples from two myocytes are shown. A, B, C: data from one myocyte. D, E, F: data from a second myocyte. Families of currents after swelling in 0.7T solution (A and D), after exposure to Cd2+ (B and E), and Cd2+ -sensitive current (C and F) are shown. Cd2+ inhibited the time dependence of _I_Cl,swell at positive potentials and had a variable effect on the steady-state current. After Cd2+ block, a slowly activating outward component of current was apparent. Block of _I_Cl,swell was greatest in the second myocyte (_D_-F).
Fig. 5
Cd2+ plus Ba2+ eliminated the time dependence of membrane currents but not the stimulation of _I_Cl,swell by PP2. A: I-V relationships in the presence of 0.2 mM Cd2+ and 1 mM Ba2+ in 1T solution, after swelling in 0.7T solution for 10 min, after exposure to 10 μM PP2 for 10 min in 0.7T solution, and after addition of 10 μM tamoxifen for 10 min to block _I_Cl,swell. B, C, D, and E: families of currents in 1T solution, 0.7T solution, 0.7T solution plus PP2, and 0.7T solution plus PP2 and tamoxifen, respectively. PP2 stimulated an outwardly rectifying, tamoxifen-sensitive current that reversed near _E_Cl.
Fig. 6
Cd2+ plus Ba2+ eliminated the time dependence of _I_Cl,swell at strongly positive potentials. To test whether Cd2+ (0.2 mM) and Ba2+ (1 mM) shifted the onset of _I_Cl,swell inactivation to more positive voltages, the membrane voltage was stepped from −60 mV to potentials between −60 and +100 mV. A: I-V relationships in 1T solution and after 10 min of swelling in 0.7T solution. B and C: families of currents in 1T and 0.7T solutions, respectively. D: swelling-induced difference current. _I_Cl,swell remained time independent to at least +100 mV.
Fig. 7
Cl− currents in symmetrical high- Cl− solutions (intracellular and extracellular Cl− concentrations = 98.6 mM). A: I-V relationships for currents in 1T solution, after 10 min of swelling in 0.7T solution, and after 10 min of exposure to 10 μM PP2 in 0.7T solution. B, C, and D: families of currents in 1T, 0.7T, and 0.7T plus PP2 solution, respectively. Swelling and PP2 stimulated outwardly rectifying, time-independent currents that reversed near 0 mV in symmetrical Cl− solutions with 0.2 mM Cd2+ and 1 mM Ba2+ in the bath.
Fig. 8
Block of protein tyrosine phosphatase (PTP) by orthovanadate partially inhibits _I_Cl,swell and prevents stimulation of _I_Cl,swell upon block of Src by PP2. A: I-V relationships for currents in 1T solution, after 10 min of swelling in 0.7T solution, after exposure to 1 mM orthovanadate in 0.7T solution for 10 min (OV), and after addition of 10 μM PP2 for 10 min. B, C, D, and E: families of currents in 1T, 0.7T, 0.7T plus orthovanadate, and 0.7T plus orthovanadate and PP2 solution, respectively.
Fig. 9
Block of epidermal growth factor receptor (EGFR) kinase by PD-153035 inhibits _I_Cl,swell. A: I-V relationships for currents in 1T solution, after 10 min of swelling in 0.7T solution, and after exposure to 20 nM PD-153035 in 0.7T solution for 12–15 min. B, C, and D: families of currents in 1T, 0.7T, and 0.7T plus PD-153035 solution, respectively.
Similar articles
- Differential effects of tyrosine kinase inhibitors on volume-sensitive chloride current in human atrial myocytes: evidence for dual regulation by Src and EGFR kinases.
Du XL, Gao Z, Lau CP, Chiu SW, Tse HF, Baumgarten CM, Li GR. Du XL, et al. J Gen Physiol. 2004 Apr;123(4):427-39. doi: 10.1085/jgp.200409013. Epub 2004 Mar 15. J Gen Physiol. 2004. PMID: 15024039 Free PMC article. - Stretch of beta 1 integrin activates an outwardly rectifying chloride current via FAK and Src in rabbit ventricular myocytes.
Browe DM, Baumgarten CM. Browe DM, et al. J Gen Physiol. 2003 Dec;122(6):689-702. doi: 10.1085/jgp.200308899. Epub 2003 Nov 10. J Gen Physiol. 2003. PMID: 14610020 Free PMC article. - Regulatory role of tyrosine phosphorylation in the swelling-activated chloride current in isolated rabbit articular chondrocytes.
Okumura N, Imai S, Toyoda F, Isoya E, Kumagai K, Matsuura H, Matsusue Y. Okumura N, et al. J Physiol. 2009 Aug 1;587(Pt 15):3761-76. doi: 10.1113/jphysiol.2009.174177. Epub 2009 Jun 15. J Physiol. 2009. PMID: 19528252 Free PMC article. - Regulation of swelling-activated Cl(-) current by angiotensin II signalling and NADPH oxidase in rabbit ventricle.
Ren Z, Raucci FJ Jr, Browe DM, Baumgarten CM. Ren Z, et al. Cardiovasc Res. 2008 Jan;77(1):73-80. doi: 10.1093/cvr/cvm031. Epub 2007 Oct 4. Cardiovasc Res. 2008. PMID: 18006461 Free PMC article. - Regulation of a swelling-activated chloride current in bovine endothelium by protein tyrosine phosphorylation and G proteins.
Voets T, Manolopoulos V, Eggermont J, Ellory C, Droogmans G, Nilius B. Voets T, et al. J Physiol. 1998 Jan 15;506 ( Pt 2)(Pt 2):341-52. doi: 10.1111/j.1469-7793.1998.341bw.x. J Physiol. 1998. PMID: 9490863 Free PMC article.
Cited by
- Caveolae act as membrane reserves which limit mechanosensitive I(Cl,swell) channel activation during swelling in the rat ventricular myocyte.
Kozera L, White E, Calaghan S. Kozera L, et al. PLoS One. 2009 Dec 14;4(12):e8312. doi: 10.1371/journal.pone.0008312. PLoS One. 2009. PMID: 20011535 Free PMC article. - Tyrosine kinase and phosphatase regulation of slow delayed-rectifier K+ current in guinea-pig ventricular myocytes.
Missan S, Linsdell P, McDonald TF. Missan S, et al. J Physiol. 2006 Jun 1;573(Pt 2):469-82. doi: 10.1113/jphysiol.2005.104422. Epub 2006 Mar 31. J Physiol. 2006. PMID: 16581870 Free PMC article. - 'Pressure-flow'-triggered intracellular Ca2+ transients in rat cardiac myocytes: possible mechanisms and role of mitochondria.
Belmonte S, Morad M. Belmonte S, et al. J Physiol. 2008 Mar 1;586(5):1379-97. doi: 10.1113/jphysiol.2007.149294. Epub 2008 Jan 10. J Physiol. 2008. PMID: 18187469 Free PMC article. - EGFR kinase regulates volume-sensitive chloride current elicited by integrin stretch via PI-3K and NADPH oxidase in ventricular myocytes.
Browe DM, Baumgarten CM. Browe DM, et al. J Gen Physiol. 2006 Mar;127(3):237-51. doi: 10.1085/jgp.200509366. Epub 2006 Feb 13. J Gen Physiol. 2006. PMID: 16505146 Free PMC article. - Nicotinic Acetylcholine Receptor (nAChR) Dependent Chorda Tympani Taste Nerve Responses to Nicotine, Ethanol and Acetylcholine.
Ren ZJ, Mummalaneni S, Qian J, Baumgarten CM, DeSimone JA, Lyall V. Ren ZJ, et al. PLoS One. 2015 Jun 3;10(6):e0127936. doi: 10.1371/journal.pone.0127936. eCollection 2015. PLoS One. 2015. PMID: 26039516 Free PMC article.
References
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. - PubMed
- Bahinski A, Nairn AC, Greengard P, Gadsby DC. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989;340:718–721. - PubMed
- Baumgarten CM, Clemo HF. Swelling-activated chloride channels in cardiac physiology and pathophysiology. Prog Biophys Mol Biol. 2003;82:25–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL046764/HL/NHLBI NIH HHS/United States
- R01 HL046764-09/HL/NHLBI NIH HHS/United States
- HL-46764/HL/NHLBI NIH HHS/United States
- R01 HL065435-04/HL/NHLBI NIH HHS/United States
- R01 HL065435-03/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous