Pro-NGF isolated from the human brain affected by Alzheimer's disease induces neuronal apoptosis mediated by p75NTR - PubMed (original) (raw)
Pro-NGF isolated from the human brain affected by Alzheimer's disease induces neuronal apoptosis mediated by p75NTR
Carlos E Pedraza et al. Am J Pathol. 2005 Feb.
Abstract
The pro-form of nerve growth factor (pro-NGF) has been shown to be a high affinity ligand for p75NTR and to induce apoptosis through this receptor. It has been reported that pro-NGF, rather than mature NGF, is the predominant form of this neurotrophin in human brain. In the present work we studied the potential involvement of pro-NGF purified from human brains affected by Alzheimer's disease (AD), where it is especially abundant, in the neuronal apoptosis observed in this disease. Western blot analysis of human brain tissue showed the existence of several pro-NGF forms. Some of these pro-NGF forms were significantly increased in AD brain cortex in a disease stage-dependent manner. Pro-NGF, purified by chromatography from human AD brains, induced apoptotic cell death in sympathetic neurons and in a p75NTR stably transfected cell line. Blocking p75NTR in cell culture abolished neuronal apoptosis caused by pro-NGF. p75NTR-transfected cells underwent apoptosis in the presence of pro-NGF while control wild-type cells did not. Taken together, these results indicate that pro-NGF purified from AD human brains can induce apoptosis in neuronal cell cultures through its interaction with the p75NTR receptor.
Figures
Figure 1
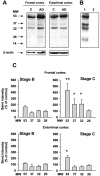
The different antibodies anti-pro-NGF and anti-mNGF recognize high molecular weight forms of pro-NGF in human brain tissue and cerebrospinal fluid. Human samples from AD brain tissue or cerebrospinal fluid (CSF) (30 μg per lane) were analyzed by Western blot using two different antibodies directed against either the pro-domain of pro-NGF (C: anti-pre-pro-NGF, Prohormone Sciences; D: anti-pro-NGF, see Materials and Methods) or the mature part of the molecule (A: H20, Santa Cruz; B: anti-mNGF, Cederlane Labs.).
Figure 2
Western blotting analysis of pro-NGF forms in human brain samples from frontal and entorhinal cortex. High molecular weight forms of pro-NGF (53, 37, 32, and 26 kd) were immunodetected with an antibody directed against the pro-domain of pro-NGF (A). Pre-incubation of anti-pro-NGF with the antigenic peptide (1:20) blocks immunoreactivity in human brain samples (B: lane 1, AD; lane 2, antigenic peptide blockage). The content of pro-NGF forms is clearly increased in Alzeimer’s disease (AD)-affected brains (A, lanes AD) compared to controls (A, lanes C). β-actin was used as loading control. Densitometry analysis of anti-pro-NGF immunodetected bands shows a significant increase of several bands in AD-affected tissue in a disease stage-dependent manner. Tissue samples obtained from human frontal and entorhinal brain cortex affected by AD were classified into A, B, and C stages according to Braak and Braak (see Table 1 and Materials and Methods). Bars represent the mean of four stage B and five stage C samples as percentage of the mean of five stage A samples (controls) (B). (*, P < 0.05; **, P < 0.01; Student’s _t_-test).
Figure 3
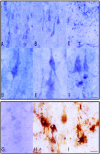
Pro-NGF immunoreactivity in the frontal cortex (A, D, and F), CA1 area of the hippocampus (B and E), and subcortical white matter of the frontal cortex (C) in control cases. Pro-NGF is expressed in neurons (A, B, D, and E) and in glia (C). Pro-NGF immunoreactivity is present as a fine granular precipitate in the cytoplasm of neurons and main dendritic branches (D and E), but also in scattered nuclei (F). H and I: Double-staining immunohistochemistry showing pro-NGF expression (dark blue precipitate) in GFAP-immunoreactive (brown) astrocytes. G: Anti-pro-NGF blocked immunoreactivity with the antigenic peptide (1:10). A–C, bar in C = 25 μm; D–F, bar in F = 10 μm. G–I, bar in I = 10 μm.
Figure 4
Analysis by Western blots of Pro-NGF isolated from frontal cortex AD-affected tissue. Protein extracts from post-mortem AD-affected tissue were processed for ion-exchange chromatography (see Materials and Methods). In the final purification fraction (hbi-pro-NGF), different pro-NGF isolated forms of 53, 32, and 26 kd (A, lane 2) were detected by Western blotting with anti-pro-NGF antibody. Hbi-pro-NGF blocks anti-pro-NGF immunodetection of pro-NGF forms in human brain tissue homogenates (B, lane 2). B, lane 1: total lysate of human brain AD-affected tissue. The 53-kd band immunodetected in hbi-pro-NGF is a glycosylated form of the pro-neurotrophin as the treatment with N-glycanase (C, lane 2) results in a decrease in its apparent MW (C, lane 1, untreated hbi-pro-NGF).
Figure 5
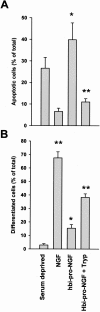
Trypsin pre-treatment of hbi-pro-NGF generates mature NGF which protects PC12 cells from deprivation-induced apoptotic death. PC12 cells were serum-deprived and treated with NGF (100 ng/ml), hbi-pro-NGF (25 ng/ml), or with trypsin-digested hbi-pro-NGF (25 ng/ml hbi-pro-NGF treated with 50 mg/ml trypsin for 10 seconds at 37°C) for 48 hours. Apoptotic nucleus morphology was detected by Hoechst staining (A). Differentiated cells were counted as positive when neurite extensions were longer than a cell body (B). Results are the mean ± SD of 900 to 1600 cells counted in a representative experiment carried out in triplicate. Statistic was done by comparing between treatments and deprived cells. **, P < 0.05, Student’s _t_-test.
Figure 6
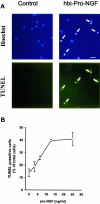
Hbi-pro-NGF induces apoptosis in SCG neurons. Cultured SCG neurons were treated for 24 hours with hbi-pro-NGF (25 ng/ml) and apoptotic cell death was evidenced by means of the TUNEL method and staining with Hoechst (A). Quantification of TUNEL-positive cells shows that the cell death increases with the increased concentration of hbi-pro-NGF (B). Bar = 50 μm.
Figure 7
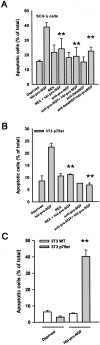
Anti-pro-NGF antibody blocks hbi-pro-NGF-induced apoptosis in SGC cells and in 3T3-p75st. Induction of apoptotic cell death in SCG cells treated with hbi-pro-NGF is prevented by pre-incubation with anti-p75 antibody (REX, 50 ng/ml), anti-pro-NGF (20 μg/ml), or anti-β-NGF (25 μg/ml) (A). Apoptotic cell death was evidenced by means of the TUNEL assay. Hbi-pro-NGF also induces apoptotic nucleus morphology determined by Hoechst staining in the non-neuronal cell line 3T3-p75st. Apoptotic nuclei reach ∼25% of total cells (B) and the pre-incubation of hbi-pro-NGF with anti-pro-NGF for 2 hours before its addition to cell cultures completely blocks cell death induced by 30-hour treatment with hbi-pro-NGF (25 ng/ml). 3T3 wild-type cells are not affected by the treatment with hbi-pro-NGF (C). **, P < 0.01, Student’s _t_-test.
Figure 8
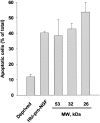
Hbi-pro-NGF forms separated by gel filtration chromatography induce cell death in 3T3-p75st cells. Three fractions of gel filtration chromatography of hbi-pro-NGF (8 μg total protein of each fraction) containing pro-NGF forms of 54, 32, and 26 kd, were added to 3T3-p75st for 30 hours. Apoptotic nuclei morphology was determined by Hoechst staining. Three μg of Hbi-pro-NGF was used as control of death induction in deprived cells. Apoptotic cell nuclei were counted as percentage of the total cells in each treatment. Bars are median ± SD of triplicates.
Similar articles
- Pro-NGF from Alzheimer's disease and normal human brain displays distinctive abilities to induce processing and nuclear translocation of intracellular domain of p75NTR and apoptosis.
Podlesniy P, Kichev A, Pedraza C, Saurat J, Encinas M, Perez B, Ferrer I, Espinet C. Podlesniy P, et al. Am J Pathol. 2006 Jul;169(1):119-31. doi: 10.2353/ajpath.2006.050787. Am J Pathol. 2006. PMID: 16816366 Free PMC article. - NGF induces apoptosis in a human neuroblastoma cell line expressing the neurotrophin receptor p75NTR.
Kuner P, Hertel C. Kuner P, et al. J Neurosci Res. 1998 Nov 15;54(4):465-74. doi: 10.1002/(SICI)1097-4547(19981115)54:4<465::AID-JNR4>3.0.CO;2-T. J Neurosci Res. 1998. PMID: 9822157 - Pro-NGF, sortilin, and p75NTR: potential mediators of injury-induced apoptosis in the mouse dorsal root ganglion.
Arnett MG, Ryals JM, Wright DE. Arnett MG, et al. Brain Res. 2007 Dec 5;1183:32-42. doi: 10.1016/j.brainres.2007.09.051. Epub 2007 Oct 26. Brain Res. 2007. PMID: 17964555 Free PMC article. - NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling.
Bradshaw RA, Pundavela J, Biarc J, Chalkley RJ, Burlingame AL, Hondermarck H. Bradshaw RA, et al. Adv Biol Regul. 2015 May;58:16-27. doi: 10.1016/j.jbior.2014.11.003. Epub 2014 Nov 20. Adv Biol Regul. 2015. PMID: 25491371 Free PMC article. Review. - The cholinergic system, nerve growth factor and the cytoskeleton.
Niewiadomska G, Mietelska-Porowska A, Mazurkiewicz M. Niewiadomska G, et al. Behav Brain Res. 2011 Aug 10;221(2):515-26. doi: 10.1016/j.bbr.2010.02.024. Epub 2010 Feb 16. Behav Brain Res. 2011. PMID: 20170684 Review.
Cited by
- proNGF Measurement in Cerebrospinal Fluid Samples of a Large Cohort of Living Patients With Alzheimer's Disease by a New Automated Immunoassay.
Malerba F, Arisi I, Florio R, Zecca C, Dell'Abate MT, Bruni Ercole B, Camerini S, Casella M, Logroscino G, Cattaneo A. Malerba F, et al. Front Aging Neurosci. 2021 Oct 27;13:741414. doi: 10.3389/fnagi.2021.741414. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34776928 Free PMC article. - Role of neurotrophic factor alterations in the neurodegenerative process in HIV associated neurocognitive disorders.
Fields J, Dumaop W, Langford TD, Rockenstein E, Masliah E. Fields J, et al. J Neuroimmune Pharmacol. 2014 Mar;9(2):102-16. doi: 10.1007/s11481-013-9520-2. Epub 2014 Feb 8. J Neuroimmune Pharmacol. 2014. PMID: 24510686 Free PMC article. Review. - Nradd Acts as a Negative Feedback Regulator of Wnt/β-Catenin Signaling and Promotes Apoptosis.
Ozalp O, Cark O, Azbazdar Y, Haykir B, Cucun G, Kucukaylak I, Alkan-Yesilyurt G, Sezgin E, Ozhan G. Ozalp O, et al. Biomolecules. 2021 Jan 14;11(1):100. doi: 10.3390/biom11010100. Biomolecules. 2021. PMID: 33466728 Free PMC article. - Neurotrophin-regulated signalling pathways.
Reichardt LF. Reichardt LF. Philos Trans R Soc Lond B Biol Sci. 2006 Sep 29;361(1473):1545-64. doi: 10.1098/rstb.2006.1894. Philos Trans R Soc Lond B Biol Sci. 2006. PMID: 16939974 Free PMC article. Review. - Age-dependent alterations in nerve growth factor (NGF)-related proteins, sortilin, and learning and memory in rats.
Terry AV Jr, Kutiyanawalla A, Pillai A. Terry AV Jr, et al. Physiol Behav. 2011 Feb 1;102(2):149-57. doi: 10.1016/j.physbeh.2010.11.005. Epub 2010 Nov 6. Physiol Behav. 2011. PMID: 21059364 Free PMC article.
References
- Duychaerts CH, Dickson DW. Neuropathology of Alzheimer’s disease. Dickson DW, editor. Basel: ISN Neuropathology Press; Neurodegenerationthe molecular pathology of dementia and movement disorders. 2003:pp 47–65.
- Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399:A23–A31. - PubMed
- Troy CM, Rabacchi SA, Xu Z, Maroney AC, Connors TJ, Shelanski ML, Greene LA. Beta amyloid-induced neuronal apoptosis requires c-Jun N-terminal kinase activation. J Neurochem. 2001;77:157–164. - PubMed
- Tsukamoto E, Hashimoto Y, Kanekura K, Niikura T, Aiso S, Nishimoto I. Characterization of the toxic mechanism triggered by Alzheimer’s amyloid-beta peptides via p75 neurotrophin receptor in neuronal hybrid cells. J Neurosci Res. 2003;73:627–636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources