Interaction between Smad7 and beta-catenin: importance for transforming growth factor beta-induced apoptosis - PubMed (original) (raw)
Interaction between Smad7 and beta-catenin: importance for transforming growth factor beta-induced apoptosis
Sofia Edlund et al. Mol Cell Biol. 2005 Feb.
Abstract
Members of the transforming growth factor beta (TGF-beta) and Wnt/wingless superfamilies regulate cell fate during development and tissue maintenance. Here we report that Smad7 interacts with beta-catenin and lymphoid enhancer binding factor 1/T-cell-specific factor (LEF1/TCF), transcriptional regulators in Wnt signaling, in a TGF-beta-dependent manner. Smad7 was found to be required for TGF-beta1-induced accumulation of beta-catenin and LEF1 in human prostate cancer (PC-3U) cells as well as in human keratinocytes (HaCaT cells). Moreover, when the endogenous Smad7 was repressed by specific small interfering RNA, TGF-beta-induced increase of activated p38, Akt phosphorylated on Ser473, glycogen synthase kinase 3beta phosphorylated on Ser9 was prevented, as well as the TGF-beta-induced association between beta-catenin and LEF1. Notably, the observed physical association of Smad7 and beta-catenin was found to be important for TGF-beta-induced apoptosis, since suppression of beta-catenin expression by small interfering RNA decreased the apoptotic response to TGF-beta.
Figures
FIG. 1.
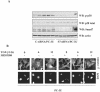
TGF-β-induced association between β-catenin, LEF1, and Smad7. (A) Coprecipitation of endogenous β-catenin with wild-type (wt) or different Flag-Smad7 deletion mutants in transiently transfected COS1 cells. Lysates of transfected cells were subjected to immunoprecipitation with Flag M2 (F) or nonimmune, control (C) antibodies and then analyzed for coprecipitated Flag-Smad7 by immunoblotting with a β-catenin antibody. (B) Coprecipitation of HA-LEF1 or HA-TCF-4 with wild-type Flag-Smad7 or a Flag-Smad7 deletion mutant (delta-N terminal; C) in transiently transfected 293T cells. Lysates of transfected cells were subjected to immunoprecipitation with polyclonal Flag antibodies and then analyzed for coprecipitated HA-LEF1/HA-TCF-4 by immunoblotting with a LEF1/TCF-4 mouse monoclonal antibody. (C and D) Coprecipitation of endogenous β-catenin and LEF1/TCF with endogenous Smad7 in PC-3U or PC-3U/AS-S7 cells treated with TGF-β for the indicated time periods. Lysates from cells were subjected to immunoprecipitation with antisera against Smad7 or control antibodies (C) and then analyzed by immunoblotting for coprecipitated β-catenin (C) or coprecipitated LEF1 (D). Immunoblots from the corresponding total cell lysate were incubated with antibodies specific for β-catenin, Smad7, LEF1/TCF-4, c-Myc, or actin. Lysates from PC-3U cells treated with TGF-β in the absence or presence of cycloheximide for the indicated time periods were investigated for the amount of endogenous c-Myc (E) or LEF1 (F). The same filters were stripped and reprobed with antibodies against actin to confirm equal loading of proteins.
FIG. 1.
TGF-β-induced association between β-catenin, LEF1, and Smad7. (A) Coprecipitation of endogenous β-catenin with wild-type (wt) or different Flag-Smad7 deletion mutants in transiently transfected COS1 cells. Lysates of transfected cells were subjected to immunoprecipitation with Flag M2 (F) or nonimmune, control (C) antibodies and then analyzed for coprecipitated Flag-Smad7 by immunoblotting with a β-catenin antibody. (B) Coprecipitation of HA-LEF1 or HA-TCF-4 with wild-type Flag-Smad7 or a Flag-Smad7 deletion mutant (delta-N terminal; C) in transiently transfected 293T cells. Lysates of transfected cells were subjected to immunoprecipitation with polyclonal Flag antibodies and then analyzed for coprecipitated HA-LEF1/HA-TCF-4 by immunoblotting with a LEF1/TCF-4 mouse monoclonal antibody. (C and D) Coprecipitation of endogenous β-catenin and LEF1/TCF with endogenous Smad7 in PC-3U or PC-3U/AS-S7 cells treated with TGF-β for the indicated time periods. Lysates from cells were subjected to immunoprecipitation with antisera against Smad7 or control antibodies (C) and then analyzed by immunoblotting for coprecipitated β-catenin (C) or coprecipitated LEF1 (D). Immunoblots from the corresponding total cell lysate were incubated with antibodies specific for β-catenin, Smad7, LEF1/TCF-4, c-Myc, or actin. Lysates from PC-3U cells treated with TGF-β in the absence or presence of cycloheximide for the indicated time periods were investigated for the amount of endogenous c-Myc (E) or LEF1 (F). The same filters were stripped and reprobed with antibodies against actin to confirm equal loading of proteins.
FIG. 1.
TGF-β-induced association between β-catenin, LEF1, and Smad7. (A) Coprecipitation of endogenous β-catenin with wild-type (wt) or different Flag-Smad7 deletion mutants in transiently transfected COS1 cells. Lysates of transfected cells were subjected to immunoprecipitation with Flag M2 (F) or nonimmune, control (C) antibodies and then analyzed for coprecipitated Flag-Smad7 by immunoblotting with a β-catenin antibody. (B) Coprecipitation of HA-LEF1 or HA-TCF-4 with wild-type Flag-Smad7 or a Flag-Smad7 deletion mutant (delta-N terminal; C) in transiently transfected 293T cells. Lysates of transfected cells were subjected to immunoprecipitation with polyclonal Flag antibodies and then analyzed for coprecipitated HA-LEF1/HA-TCF-4 by immunoblotting with a LEF1/TCF-4 mouse monoclonal antibody. (C and D) Coprecipitation of endogenous β-catenin and LEF1/TCF with endogenous Smad7 in PC-3U or PC-3U/AS-S7 cells treated with TGF-β for the indicated time periods. Lysates from cells were subjected to immunoprecipitation with antisera against Smad7 or control antibodies (C) and then analyzed by immunoblotting for coprecipitated β-catenin (C) or coprecipitated LEF1 (D). Immunoblots from the corresponding total cell lysate were incubated with antibodies specific for β-catenin, Smad7, LEF1/TCF-4, c-Myc, or actin. Lysates from PC-3U cells treated with TGF-β in the absence or presence of cycloheximide for the indicated time periods were investigated for the amount of endogenous c-Myc (E) or LEF1 (F). The same filters were stripped and reprobed with antibodies against actin to confirm equal loading of proteins.
FIG. 2.
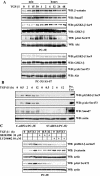
Smad7 is required for TGF-β-induced association between β-catenin and LEF1. (A) Lysates from PC-3U (upper panel) and HaCaT cells (lower panel), transiently transfected with control (C-siRNA) or specific Smad7 siRNAs (S7-siRNA), were treated or not with TGF-β for the indicated time periods and the total cell lysates were subjected to coimmunoprecipitation with a polyclonal antibody for β-catenin or a nonimmune, control (C) antibody and then analyzed for coprecipitated LEF1. Cell lysates from 293T cells transiently transfected with HA-LEF1 were used as a positive control (Pos.Ctrl.) to verify the identity of endogenous LEF1. The cell lysates were investigated for the amount of endogenous LEF1, β-catenin, and Smad7. The filters were then stripped, blocked, and reprobed with actin antibodies to confirm equal loading of proteins. (B) The same cell lysates from PC-3U and HaCaT cells were also investigated for the amount of endogenous Smad7 and c-Myc by immunoblotting. Total cell lysates from cells ectopically expressing Flag-Smad7 (PC-3U/pMEP4-Flag-S7) were used as positive control for Smad7 (Pos. Ctrl). The filters then were stripped, blocked, and reprobed with actin antibodies to confirm equal loading of proteins.
FIG. 3.
Smad7 is required for the TGF-β-induced redistribution of β-catenin to the perinuclear-nuclear compartment. (A) Subcellular localization of endogenous Smad7 and β-catenin in PC-3U or PC-3U/AS-S7 cells after treatment with TGF-β for 2 h, 6 h, or 12 h as investigated by immunofluorescence with antibodies against Smad7 and β-catenin, together with additional staining of the nuclei by DAPI. Note that β-catenin is localized in the cytoplasm and in cell-cell contacts in untreated cells. After 2 h of TGF-β treatment, a portion of Smad7 is perinuclear or nuclear as β-catenin. At longer time points (12 h) after TGF-β treatment, both proteins predominantly localize in the perinuclear and nuclear compartment (left panel). In contrast, in PC-3U/AS-S7 cells, where the levels of endogenous Smad7 is reduced, no significant change of the subcellular localization of β-catenin was observed (right panel). (B) Quantification of the effect of TGF-β treatment on the subcellular localization of endogenous β-catenin in PC-3U and PC-3U/AS-S7 cells is shown in Fig. 2A. (C) PC-3U cells were transiently transfected with control (C) or specific Smad7 (S7) siRNAs and treated or not with TGF-β for the indicated times. The subcellular localizations of β-catenin and Smad7 were analyzed as described above. The values for the quantification of the subcellular localization of β-catenin in the transfected cells are shown in panel D. (F) HaCaT cells were transiently transfected with control (C) or specific Smad7 (S7) siRNAs and treated or not for the indicated time periods. The distribution of endogenous β-catenin and Smad7 was investigated by immunofluorescence and shown in panel E. The quantification of the differences in distribution of β-catenin is shown in panel F. Approximately 300 cells from each condition were counted under the microscope at 40× magnification. (G) Immunoblots from subcellular fractions of PC-3U and HaCat; nucleus (N) and cytoplasm (CP), derived from cells treated or not with TGF-β for the indicated time periods. Lamin A and β-tubulin were used as markers for the nucleus and cytoplasmic fractions, respectively. Total cell lysate from cells ectopically expressing Flag-Smad7 (PC-3U/pMEP4-Flag-S7) were used as the positive control for Smad7.
FIG. 3.
Smad7 is required for the TGF-β-induced redistribution of β-catenin to the perinuclear-nuclear compartment. (A) Subcellular localization of endogenous Smad7 and β-catenin in PC-3U or PC-3U/AS-S7 cells after treatment with TGF-β for 2 h, 6 h, or 12 h as investigated by immunofluorescence with antibodies against Smad7 and β-catenin, together with additional staining of the nuclei by DAPI. Note that β-catenin is localized in the cytoplasm and in cell-cell contacts in untreated cells. After 2 h of TGF-β treatment, a portion of Smad7 is perinuclear or nuclear as β-catenin. At longer time points (12 h) after TGF-β treatment, both proteins predominantly localize in the perinuclear and nuclear compartment (left panel). In contrast, in PC-3U/AS-S7 cells, where the levels of endogenous Smad7 is reduced, no significant change of the subcellular localization of β-catenin was observed (right panel). (B) Quantification of the effect of TGF-β treatment on the subcellular localization of endogenous β-catenin in PC-3U and PC-3U/AS-S7 cells is shown in Fig. 2A. (C) PC-3U cells were transiently transfected with control (C) or specific Smad7 (S7) siRNAs and treated or not with TGF-β for the indicated times. The subcellular localizations of β-catenin and Smad7 were analyzed as described above. The values for the quantification of the subcellular localization of β-catenin in the transfected cells are shown in panel D. (F) HaCaT cells were transiently transfected with control (C) or specific Smad7 (S7) siRNAs and treated or not for the indicated time periods. The distribution of endogenous β-catenin and Smad7 was investigated by immunofluorescence and shown in panel E. The quantification of the differences in distribution of β-catenin is shown in panel F. Approximately 300 cells from each condition were counted under the microscope at 40× magnification. (G) Immunoblots from subcellular fractions of PC-3U and HaCat; nucleus (N) and cytoplasm (CP), derived from cells treated or not with TGF-β for the indicated time periods. Lamin A and β-tubulin were used as markers for the nucleus and cytoplasmic fractions, respectively. Total cell lysate from cells ectopically expressing Flag-Smad7 (PC-3U/pMEP4-Flag-S7) were used as the positive control for Smad7.
FIG. 3.
Smad7 is required for the TGF-β-induced redistribution of β-catenin to the perinuclear-nuclear compartment. (A) Subcellular localization of endogenous Smad7 and β-catenin in PC-3U or PC-3U/AS-S7 cells after treatment with TGF-β for 2 h, 6 h, or 12 h as investigated by immunofluorescence with antibodies against Smad7 and β-catenin, together with additional staining of the nuclei by DAPI. Note that β-catenin is localized in the cytoplasm and in cell-cell contacts in untreated cells. After 2 h of TGF-β treatment, a portion of Smad7 is perinuclear or nuclear as β-catenin. At longer time points (12 h) after TGF-β treatment, both proteins predominantly localize in the perinuclear and nuclear compartment (left panel). In contrast, in PC-3U/AS-S7 cells, where the levels of endogenous Smad7 is reduced, no significant change of the subcellular localization of β-catenin was observed (right panel). (B) Quantification of the effect of TGF-β treatment on the subcellular localization of endogenous β-catenin in PC-3U and PC-3U/AS-S7 cells is shown in Fig. 2A. (C) PC-3U cells were transiently transfected with control (C) or specific Smad7 (S7) siRNAs and treated or not with TGF-β for the indicated times. The subcellular localizations of β-catenin and Smad7 were analyzed as described above. The values for the quantification of the subcellular localization of β-catenin in the transfected cells are shown in panel D. (F) HaCaT cells were transiently transfected with control (C) or specific Smad7 (S7) siRNAs and treated or not for the indicated time periods. The distribution of endogenous β-catenin and Smad7 was investigated by immunofluorescence and shown in panel E. The quantification of the differences in distribution of β-catenin is shown in panel F. Approximately 300 cells from each condition were counted under the microscope at 40× magnification. (G) Immunoblots from subcellular fractions of PC-3U and HaCat; nucleus (N) and cytoplasm (CP), derived from cells treated or not with TGF-β for the indicated time periods. Lamin A and β-tubulin were used as markers for the nucleus and cytoplasmic fractions, respectively. Total cell lysate from cells ectopically expressing Flag-Smad7 (PC-3U/pMEP4-Flag-S7) were used as the positive control for Smad7.
FIG. 3.
Smad7 is required for the TGF-β-induced redistribution of β-catenin to the perinuclear-nuclear compartment. (A) Subcellular localization of endogenous Smad7 and β-catenin in PC-3U or PC-3U/AS-S7 cells after treatment with TGF-β for 2 h, 6 h, or 12 h as investigated by immunofluorescence with antibodies against Smad7 and β-catenin, together with additional staining of the nuclei by DAPI. Note that β-catenin is localized in the cytoplasm and in cell-cell contacts in untreated cells. After 2 h of TGF-β treatment, a portion of Smad7 is perinuclear or nuclear as β-catenin. At longer time points (12 h) after TGF-β treatment, both proteins predominantly localize in the perinuclear and nuclear compartment (left panel). In contrast, in PC-3U/AS-S7 cells, where the levels of endogenous Smad7 is reduced, no significant change of the subcellular localization of β-catenin was observed (right panel). (B) Quantification of the effect of TGF-β treatment on the subcellular localization of endogenous β-catenin in PC-3U and PC-3U/AS-S7 cells is shown in Fig. 2A. (C) PC-3U cells were transiently transfected with control (C) or specific Smad7 (S7) siRNAs and treated or not with TGF-β for the indicated times. The subcellular localizations of β-catenin and Smad7 were analyzed as described above. The values for the quantification of the subcellular localization of β-catenin in the transfected cells are shown in panel D. (F) HaCaT cells were transiently transfected with control (C) or specific Smad7 (S7) siRNAs and treated or not for the indicated time periods. The distribution of endogenous β-catenin and Smad7 was investigated by immunofluorescence and shown in panel E. The quantification of the differences in distribution of β-catenin is shown in panel F. Approximately 300 cells from each condition were counted under the microscope at 40× magnification. (G) Immunoblots from subcellular fractions of PC-3U and HaCat; nucleus (N) and cytoplasm (CP), derived from cells treated or not with TGF-β for the indicated time periods. Lamin A and β-tubulin were used as markers for the nucleus and cytoplasmic fractions, respectively. Total cell lysate from cells ectopically expressing Flag-Smad7 (PC-3U/pMEP4-Flag-S7) were used as the positive control for Smad7.
FIG. 4.
Smad7 is required for TGF-β-induced phosphorylation of p38 and the TGF-β-induced nuclear accumulation of β-catenin is associated with p38. (A) Lysates from PC-3U cells transiently transfected with control siRNA (C-siRNA) or specific Smad7 siRNA (S7-siRNA) were treated or not with TGF-β for the indicated time periods and the total cell lysates were investigated for the amount of endogenous p-p38 and Smad7. The filters were then stripped and reprobed with antibodies against total p38 to show the specificity of the band for p38 and again stripped and reprobed with actin antibodies to confirm equal loading of proteins. Total cell lysates from cells ectopically expressing Flag-Smad7 (PC-3U/pMEP4S-7) were used as the positive control for Smad7 (Ctrl). (B) The subcellular localization of β-catenin in PC-3U cells treated with TGF-β in the absence or presence of 10 μM SB203580, the p38 inhibitor, for the indicated time periods were analyzed by immunofluorescence staining. Note the absence of TGF-β-induced redistribution in cells simultaneously treated with SB203580. SB203580 was added to cells 1 h before the initiation of the experiment. Nuclei were counterstained with DAPI. (C) Mouse embryonic fibroblasts derived from wild-type (wt) or p38α−/− knockout mice were treated with TGF-β for the indicated time periods and subjected to immunofluorescence staining for endogenous β-catenin and Smad7. Nuclei were counterstained with DAPI. Note the lack of TGF-β-induced effects on β-catenin and Smad7 colocalization in p38α−/− mouse embryonic fibroblasts compared to wild-type mouse embryonic fibroblasts.
FIG. 4.
Smad7 is required for TGF-β-induced phosphorylation of p38 and the TGF-β-induced nuclear accumulation of β-catenin is associated with p38. (A) Lysates from PC-3U cells transiently transfected with control siRNA (C-siRNA) or specific Smad7 siRNA (S7-siRNA) were treated or not with TGF-β for the indicated time periods and the total cell lysates were investigated for the amount of endogenous p-p38 and Smad7. The filters were then stripped and reprobed with antibodies against total p38 to show the specificity of the band for p38 and again stripped and reprobed with actin antibodies to confirm equal loading of proteins. Total cell lysates from cells ectopically expressing Flag-Smad7 (PC-3U/pMEP4S-7) were used as the positive control for Smad7 (Ctrl). (B) The subcellular localization of β-catenin in PC-3U cells treated with TGF-β in the absence or presence of 10 μM SB203580, the p38 inhibitor, for the indicated time periods were analyzed by immunofluorescence staining. Note the absence of TGF-β-induced redistribution in cells simultaneously treated with SB203580. SB203580 was added to cells 1 h before the initiation of the experiment. Nuclei were counterstained with DAPI. (C) Mouse embryonic fibroblasts derived from wild-type (wt) or p38α−/− knockout mice were treated with TGF-β for the indicated time periods and subjected to immunofluorescence staining for endogenous β-catenin and Smad7. Nuclei were counterstained with DAPI. Note the lack of TGF-β-induced effects on β-catenin and Smad7 colocalization in p38α−/− mouse embryonic fibroblasts compared to wild-type mouse embryonic fibroblasts.
FIG. 5.
TGF-β regulates the levels of β-catenin in PC-3U cells via phosphorylation of GSK-3β on Ser9 and Akt on Ser473 in a Smad7-, p38 MAP kinase-, and phosphatidylinositol 3-kinase-dependent manner. Total cell lysates were prepared from PC-3U and PC-3U/AS-S7 cells, treated or not with TGF-β, and used for immunoblotting. (A) Levels of β-catenin and Smad7 and of GSK-3β and Akt phosphorylated at Ser9 and Ser473, respectively, in PC-3U cells treated with TGF-β for different time periods. In the upper and lower panels, the phosphorylated and nonphosphorylated forms, respectively, of GSK-3β and Akt, are shown. A similar analysis with PC-3U/AS-S7 cells treated with TGF-β is also shown. Equal amounts of proteins were loaded for each cell line. The filters were subjected to immunoblotting simultaneously under equal conditions. (B) Lysates from PC-3U cells transiently transfected with control (C-siRNA) or specific Smad7 siRNAs (S7-siRNA) were treated or not with TGF-β for the indicated time periods, and the total cell lysates were investigated for the amount of endogenous pGSK-3β Ser9, phospho-Akt Ser473, and Smad7. The filters were then stripped and reprobed with actin antibodies to confirm equal loading of proteins. Total cell lysate from cells ectopically expressing Flag-Smad7 (PC-3U/pMEP4S-7) were used as the positive control for Smad7 (Pos. Ctrl). (C) Effects of the p38 inhibitor SB203580 and the phosphatidylinositol 3-kinase inhibitor LY294002 on TGF-β-induced phosphorylation of GSK-3β on Ser9. Total cell lysates were prepared from PC-3U cells treated or not with TGF-β, in the presence or absence of SB203580 and LY294002, andsubjected to immunoblotting. The phosphorylation of GSK-3β on Ser9 and amount of β-catenin are shown in the upper two panels, and the phosphorylation of Akt on Ser473 is shown in the lower panel. Both filters were stripped, blocked, and reprobed with actin antibodies, which served as the control for equal loading of proteins.
FIG. 6.
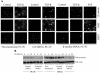
Transfection of siRNA for β-catenin reduces TGF-β induced apoptosis. (A) PC-3U and HaCaT cells were transfected or not with specific or unspecific (ctrl) siRNA for β-catenin and treated with TGF-β for 24 h. The localization and amount of β-catenin and number of apoptotic cells, shown by M30 staining, were examined by immunofluorescence. Nuclei were counterstained with DAPI. PC-3U cells transfected with specific siRNA for β-catenin and treated with 1 μM staurosporine (STS) for 8 h served as the control. The number of apoptotic cells was determined in triplicate samples in three different experiments, and at least 1,000 nuclei/sample were counted. The mean values are given in Table 1. Note the correlation between M30 staining and high levels of β-catenin. (B) Lysates from PC-3U and HaCaT cells transiently transfected with control (Ctrl) siRNA or specific β-catenin siRNA were treated or not with TGF-β for the indicated time periods, and the total cell lysates were investigated for the amount of endogenous β-catenin. The filters were then stripped and reprobed with actin antibodies to confirm equal loading of proteins.
FIG. 7.
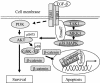
Schematic illustration of the molecular mechanisms involved in the TGF-β-Smad7 signaling pathway resulting in activation of Akt and p38, followed by phosphorylation and inactivation of GSK-3β. The inactivated GSK-3β thus cannot phosphorylate and cause degradation of β-catenin, which leads to its accumulation. The figure summarizes the result of the current study and of a previously published study (18). For further explanation and details, see the text.
Similar articles
- Transforming growth factor-beta1 (TGF-beta)-induced apoptosis of prostate cancer cells involves Smad7-dependent activation of p38 by TGF-beta-activated kinase 1 and mitogen-activated protein kinase kinase 3.
Edlund S, Bu S, Schuster N, Aspenström P, Heuchel R, Heldin NE, ten Dijke P, Heldin CH, Landström M. Edlund S, et al. Mol Biol Cell. 2003 Feb;14(2):529-44. doi: 10.1091/mbc.02-03-0037. Mol Biol Cell. 2003. PMID: 12589052 Free PMC article. - Activation of the {beta}-catenin/T-cell-specific transcription factor/lymphoid enhancer factor-1 pathway by plasminogen activators in ECV304 carcinoma cells.
Maupas-Schwalm F, Robinet C, Augé N, Thiers JC, Garcia V, Cambus JP, Salvayre R, Nègre-Salvayre A. Maupas-Schwalm F, et al. Cancer Res. 2005 Jan 15;65(2):526-32. Cancer Res. 2005. PMID: 15695395 - 2-Methoxyestradiol-induced apoptosis in prostate cancer cells requires Smad7.
Davoodpour P, Landström M. Davoodpour P, et al. J Biol Chem. 2005 Apr 15;280(15):14773-9. doi: 10.1074/jbc.M414470200. Epub 2005 Feb 11. J Biol Chem. 2005. PMID: 15708859 - Regulation of the versican promoter by the beta-catenin-T-cell factor complex in vascular smooth muscle cells.
Rahmani M, Read JT, Carthy JM, McDonald PC, Wong BW, Esfandiarei M, Si X, Luo Z, Luo H, Rennie PS, McManus BM. Rahmani M, et al. J Biol Chem. 2005 Apr 1;280(13):13019-28. doi: 10.1074/jbc.M411766200. Epub 2005 Jan 24. J Biol Chem. 2005. PMID: 15668231 - Transcriptional regulation by Smads: crosstalk between the TGF-beta and Wnt pathways.
Letamendia A, Labbé E, Attisano L. Letamendia A, et al. J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 1):S31-9. J Bone Joint Surg Am. 2001. PMID: 11263663 Review.
Cited by
- The hormetic morphogen theory of curvature and the morphogenesis and pathology of tubular and other curved structures.
Fosslien E. Fosslien E. Dose Response. 2009 Oct 16;7(4):307-31. doi: 10.2203/dose-response.09-013.Fosslien. Dose Response. 2009. PMID: 20011651 Free PMC article. - Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure.
Wang WH, McNatt LG, Pang IH, Millar JC, Hellberg PE, Hellberg MH, Steely HT, Rubin JS, Fingert JH, Sheffield VC, Stone EM, Clark AF. Wang WH, et al. J Clin Invest. 2008 Mar;118(3):1056-64. doi: 10.1172/JCI33871. J Clin Invest. 2008. PMID: 18274669 Free PMC article. - Unexpected activities of Smad7 in Xenopus mesodermal and neural induction.
de Almeida I, Rolo A, Batut J, Hill C, Stern CD, Linker C. de Almeida I, et al. Mech Dev. 2008 May-Jun;125(5-6):421-31. doi: 10.1016/j.mod.2008.02.002. Epub 2008 Feb 12. Mech Dev. 2008. PMID: 18359614 Free PMC article. - Activation of beta-Catenin in mouse prostate causes HGPIN and continuous prostate growth after castration.
Yu X, Wang Y, Jiang M, Bierie B, Roy-Burman P, Shen MM, Taketo MM, Wills M, Matusik RJ. Yu X, et al. Prostate. 2009 Feb 15;69(3):249-62. doi: 10.1002/pros.20877. Prostate. 2009. PMID: 18991257 Free PMC article. - SMAD7: a timer of tumor progression targeting TGF-β signaling.
Luo L, Li N, Lv N, Huang D. Luo L, et al. Tumour Biol. 2014 Sep;35(9):8379-85. doi: 10.1007/s13277-014-2203-7. Epub 2014 Jun 17. Tumour Biol. 2014. PMID: 24935472 Review.
References
- Ahmed, Y., S. Hayashi, A. Levine, and E..Wieschau. 1998. Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell 93:1171-1182. - PubMed
- Allard,, S., P. Adin, L. Gouedard, N. Clemente, N. Josso, Orgebin- C. Crist, J. Picard, and F. Xavier. 2000. Molecular mechanisms of hormone-mediated Mullerian duct regression: involvement of β-catenin. Development 127:3349-3360. - PubMed
- Bai, S., and X. Cao. 2002. A nuclear antagonistic mechanism of inhibitory Smads in transforming growth factor-beta signaling. J. Biol. Chem. 277:4176-4182. - PubMed
- Bai, S., X. Shi, X. Yang, and X. Cao. 2000. Smad6 as a transcriptional corepressor. J. Biol. Chem. 275:8267-8270. - PubMed
- Barker, N., P. Morin, and H. Clevers. 2000. The Yin-Yang of TCF/β-catenin signaling. Adv. Cancer Res. 77:1-24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous