Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB - PubMed (original) (raw)
Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB
Dongsheng Cai et al. Nat Med. 2005 Feb.
Abstract
We show that NF-kappaB and transcriptional targets are activated in liver by obesity and high-fat diet (HFD). We have matched this state of chronic, subacute 'inflammation' by low-level activation of NF-kappaB in the liver of transgenic mice, designated LIKK, by selectively expressing constitutively active IKK-b in hepatocytes. These mice exhibit a type 2 diabetes phenotype, characterized by hyperglycemia, profound hepatic insulin resistance, and moderate systemic insulin resistance, including effects in muscle. The hepatic production of proinflammatory cytokines, including IL-6, IL-1beta and TNF-alpha, was increased in LIKK mice to a similar extent as induced by HFD in in wild-type mice. Parallel increases were observed in cytokine signaling in liver and mucscle of LIKK mice. Insulin resistance was improved by systemic neutralization of IL-6 or salicylate inhibition of IKK-beta. Hepatic expression of the IkappaBalpha superrepressor (LISR) reversed the phenotype of both LIKK mice and wild-type mice fed an HFD. These findings indicate that lipid accumulation in the liver leads to subacute hepatic 'inflammation' through NF-kappaB activation and downstream cytokine production. This causes insulin resistance both locally in liver and systemically.
Figures
Figure 1
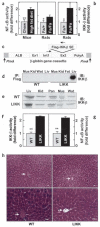
IKK-β and NF-κB activities in liver. (a) NF-κB and (b) IKK-β activities are expressed as fold differences (n = 6, *P < 0.05; **P < 0.01). (c) Schematic map of the transgene construct showing Flag-tagged IKK-β S177E,S181E subcloned into exon 2 of the Alb1 promoter-driven truncated β-globin gene vector. (d) Transgene expression and (e) total amounts of IKK-β in skeletal muscle (Mus), kidney (Kid), white adipose tissue (Wat), liver (Liv) and pancreas (Pan). (f) IKK-β and (g) NF-κB activities in liver (n = 4–6, **P < 0.01). (h) Hematoxylin and eosin–stained sections of liver. Arrows indicate central veins. Scale bars, 50 μm.
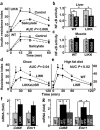
Figure 2
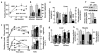
Carbohydrate metabolism. (a) Fasting insulin levels (n = 8–12; AUC, area under curve). (b) Hyperinsulinemic-euglycemic clamps were performed in 12–15-week-old male mice (n = 10, *P < 0.05). (c,d) Glucose tolerance tests were performed in 12-week-old male mice (n = 6). (c) Glucose concentrations or (d) insulin resistance index (IRI = glucose concentration (mmol/L) × insulin concentration (mU/L) ÷ 22.5) were plotted versus time (left) and as AUC (right, per 120 min). *P < 0.05, **P < 0.01. (e) Hepatic glucose production and glycogen synthesis determined during hyperinsulinemic-euglycemic clamps (n = 10; **P < 0.01). (f) GSK3β phosphorylation in liver after hyperinsulinemic-euglycemic clamps. (g) mRNA expression levels for gluconeogenic enzymes determined using real-time RT-PCR (*P < 0.05, **P < 0.01). (h) Glycogen synthesis and glucose uptake in skeletal muscle during hyperinsulinemic-euglycemic clamps (n = 10, *P < 0.05).
Figure 3
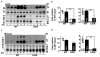
Insulin signaling in liver and skeletal muscle of LIKK mice. Fasting mice were injected with insulin (5.0 mU/g) or saline. Proteins were immunoprecipitated (IP) from (a,b) liver or (c,d) skeletal muscle (gastrocnemius) immunoblotted (IB) with the indicated antibodies. (b,d) Specific phosphorylation: open bars, without insulin; closed bars, with insulin; n = 3; *P < 0.05, **P < 0.01. Ins, insulin; IR, insulin receptor.
Figure 4
Cytokine signaling in LIKK mice. (a) Western blots of phosphorylated Stat-3 or Stat-3 in liver. (b) Circulating levels of TNF-α, IL-1β and IL-6 (n = 4–8; *P < 0.05, ** P < 0.01). (c,d) mRNA levels in skeletal muscle determined by real-time RT-PCR. NF-κB activity measured by specific DNA binding (n = 6; * P < 0.05). (e) Glucose tolerance tests 2–3 weeks after treatment with neutralizing antibody to IL-6 (αIL6) or control IgG (n = 6; insulin resistance index, IRI = glucose concentration (mmol/L) × insulin concentration (mU/L) ÷ 22.5). (f) Phosphorylated Stat-3 and Stat-3 in livers from mice treated with antibodies to IL-6 (αIL6) or control IgG. mRNA levels in liver (g) and gastrocnemius muscle (h) determined by real-time RT-PCR (*P < 0.05, ** P < 0.01). Il1b encodes IL-1β; Il1R1 encodes IL-1R1.
Figure 5
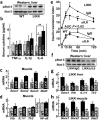
Reversal of insulin resistance and inflammation. (a) Mice (16-week-old males; n = 6–8) treated orally with high-dose salicylate were subjected to glucose tolerance testing (insulin resistance index = glucose concentration (mmol/L) × insulin concentration (mU/L) ÷ 22.5). (b,c) Binding of NF-κB to DNA in liver and skeletal muscle of control and sodium salicylate-treated (NaSal) mice. (d) Doubly transgenic LIKK × LISR mice were subjected to glucose tolerance testing (12-week-old males; n = 5 per group). (e) Mice fed a HFD for 3 months were subjected to glucose tolerance testing (n = 6–7 per group). mRNA levels of monocyte-macrophage proteins in liver of (f) chow-fed wild-type and LIKK mice and (g) wild-type and LISR mice fed chow or HFD (P < 0.05, **P < 0.01).
References
- Mokdad AH, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. - PubMed
- National Diabetes Data Group . Diabetes in America. 2nd National Institutes of Diabetes and Digestive Diseases, National Institutes of Health; Bethesda, Maryland: 1995.
- Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Invest. 2002;32(Suppl 3):14–23. - PubMed
- Seppala-Lindroos A, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J. Clin. Endocrinol. Metab. 2002;87:3023–3028. - PubMed
- Ryysy L, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000;49:749–758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK051729/DK/NIDDK NIH HHS/United States
- R37 DK051729/DK/NIDDK NIH HHS/United States
- R01 DK45493/DK/NIDDK NIH HHS/United States
- P30 DK36836/DK/NIDDK NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
- R01 DK51729/DK/NIDDK NIH HHS/United States
- R01 DK045943/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous