A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73 - PubMed (original) (raw)
A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73
Gad Asher et al. Genes Dev. 2005.
Abstract
Protein degradation is an essential and highly regulated process. The proteasomal degradation of the tumor suppressors p53 and p73 is regulated by both polyubiquitination and by an ubiquitin-independent process. Here, we show that this ubiquitin-independent process is mediated by the 20S proteasomes and is regulated by NQO1. NQO1 physically interacts with p53 and p73 in an NADH-dependent manner and protects them from 20S proteasomal degradation. Remarkably, the vast majority of NQO1 in cells is found in physical association with the 20S proteasomes, suggesting that NQO1 functions as a gatekeeper of the 20S proteasomes. We further show that this pathway plays a role in p53 accumulation in response to ionizing radiation. Our findings provide the first evidence for in vivo degradation of p53 and p73 by the 20S proteasomes and its regulation by NQO1 and NADH level.
Figures
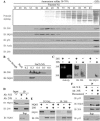
Figure 1.
NQO1 is physically associated with 20S but not 26S proteasomes. (A) Mouse liver extract was precipitated at 38%-70% ammonium sulfate and subjected to Sepharose 6B gel-filtration chromatography. Fractions were collected and analyzed by SDS-PAGE and immunoblotting (IB). (B) Fractions from Sepharose 6B gel-filtration column containing 20S proteasomes were loaded on Resource Q anion-exchange column and eluted with different concentrations of NaCl. Fractions were analyzed by SDS-PAGE and immunoblotting (IB). (C) Purified 26S and 20S proteasomes (0.3 M NaCl fraction) were subjected to nondenaturing PAGE and analyzed for peptidase activity or by immunoblotting (IB). (D) Fractions from Sepharose 6B gel-filtration column containing 20S proteasomes were pooled (Input), and immunoprecipitation experiments were performed with mouse anti-Flag antibody as a control for nonspecific binding (Ab: N.S) or with rabbit anti-C9 antibody (Ab: 20S). The immunoprecipitants (IP) and the supernatant (Sup) were analyzed by SDS-PAGE and immunoblotting (IB) (E) 35S-labeled NQO1 was incubated alone or mixed together with 20S proteasomes (TOTAL). The 20S proteasomes were immunoprecipitated with rabbit anti-C9 antibody (IP: 20S). (F) Fractions from Sepharose 6B gel-filtration column containing 20S proteasomes were pooled and immunoprecipitation experiments were performed with mouse anti-Flag antibody as a control for nonspecific binding (Ab: N.S) or with rabbit anti-C9 antibody (Ab: 20S) in the absence (-) or presence (+) of 200 μM dicoumarol. The immunoprecipitants (IP) and the supernatant (Sup) were analyzed by SDS-PAGE and immunoblotting (IB). Immunoblot analysis (IB) was performed with rabbit anti-C9 antibody to identify the 20S proteasomes, rabbit anti-TBP1 antibody to identify the 26S proteasomes, goat anti-NQO1 antibody, mouse anti-mouse p53 antibody, and with mouse anti-Actin. 35S-labeled NQO1 was detected by autoradiography. Proteasome peptidase activity was determined by their ability to hydrolyze the flurogenic peptide suc-LLVY-AMC, as described in Materials and Methods.
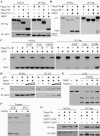
Figure 2.
NQO1 selectively protects p73α, but not p73β, from 20S proteasomal degradation. (A) 35S-labeled p53 was incubated without (-) or with (+) 20S proteasomes at 37°C for 15, 30, and 60 min, without (-) or with (+) 50 μM MG132. (B) 35S-labeled p73α was incubated without (-) or with (+) 20S proteasomes without (-) or with (+) 50 μM MG132 at 37°C for 1 h. (C) 35S-labeled Flag-p73α or immunoaffinity-purified 35S-labeled Flag-p73α were incubated without (-) or with (+) 20S proteasomes at 37°C for 1 h. (D) 35S-labeled p53 was incubated in the presence of 1 mM NADH without (-) or with (+) 20S proteasomes, without (-) or with (+) 35S-labeled NQO1 (E) 35S-labeled p73α or p73β were incubated in the presence of 1 mM NADH without (-) or with (+) 20S proteasomes, without (-) or with (+) 35S-labeled NQO1. 35S-labeled p73 and NQO1 were analyzed by SDS-PAGE and detected by autoradiography. The 20S proteasomes were detected by immunoblot analysis (IB) with rabbit anti C9 antibody. (F) Cell extracts of HCT116 cells and HCT116 cells that stably express pEFIRES NQO1 were fractionated by SDS-PAGE; immunoblot analysis (IB) was carried out with mouse anti-p53, with rabbit anti-p73 antibody, and with goat anti-NQO1 antibody.
Figure 3.
NQO1 binds the SAM domain of p73α in an NADH-dependent manner. (A) 293 HEK cells were transiently transfected with pEFIRES Flag-p73α, pEFIRES Flag-p73β, or pEFIRES Flag-p73Δ1-317 without (-) or with pEFIRES-NQO1 (TOTAL). Flag-p73 was immunoprecipitated with anti-Flag beads (IP: Flag). (B) In vitro 35S-labeled Flag-p73α or Flag-p73β were incubated alone or mixed together with 35S-labeled NQO1 (TOTAL). Flag-p73 was immunoprecipitated with anti-Flag beads (IP: Flag). (C) 35S-labeled Flag-p73α and NQO1 were incubated alone or mixed together without or with NAD+, FAD, or NADH (TOTAL). Flag-p73 was immunoprecipitated with anti-Flag beads (IP: Flag). (D) In vitro 35S-labeled p53 was incubated alone (-) or together with (+) recombinant NQO1 in the absence (-) or presence of 100 μM or 1 mM NADH. p53 was immunoprecipitated with mouse anti-human p53 antibody (IP: p53). (E)In vitro 35S-labeled Flag-p73α was incubated without (-) or with (+) 1 mM NADH, in vitro-translated NQO1 or 1 mM NADH together with in vitro-translated NQO1, and partially digested with trypsin. (F) In vitro 35S-labeled Flag-p73α or Flag-p73β was incubated without (-) or with (+) 1 mM NADH and partially digested with trypsin. (G) 293 HEK cells were transiently transfected with pEFIRES Flag-p73α alone or together with pEFIRES NQO1, pEFIRES NQO1-Y128F, or pEFIRES NQO1-Y128V (TOTAL). Flag-p73 was immunoprecipitated with anti-Flag beads (IP: Flag). Immunoblot analysis (IB) was carried out with mouse monoclonal anti-Flag antibody and with goat anti-NQO1 antibody. 35S-labeled NQO1, p53, and p73 were detected by autoradiography.
Figure 4.
Dicoumarol disrupts the binding of NQO1 to p73α and induces ubiquitin-independent proteasomal degradation of p73α.(A) 35S-labeled Flag-p73α was incubated alone or with NQO1 in the absence (-) or presence of (+) NADH or NADH together with 300 μM dicoumarol (TOTAL). Flag-p73 was immunoprecipitated with anti-Flag beads (IP: Flag). (B) 293 HEK cells were transiently transfected with pEFIRES Flag-p73β or pEFIRES Flag-p73α together with pEFIRES NQO1 (TOTAL). Flag-p73 was immunoprecipitated with anti-Flag beads (IP: Flag). The beads were washed without (-) or with (+) 300 μM dicoumarol (dicoumarol wash). (C) 35S-labeled p73α was incubated in reticulocyte lysate degradation mixture at 37°C for 90 and 180 min in the absence or presence of 300 μM dicoumarol. (D) 35S-labeled Flag-p73α was incubated alone or together with 35S-labeled NQO1 in reticulocyte lysate degradation mixture at 37°C for 90 min. (E) 35S-labeled p73α was incubated in the presence of NADH without (-) or with (+) 20S proteasomes without (-) or with (+) 35S-labeled NQO1 and without (-) or with (+) 200 μM dicoumarol. (F) HCT116 or COS 1 cells were cultured without (-) or with 200 or 400 μM dicoumarol for 5 h. (G) HCT116 cells were transiently transfected with pSG5 HA-p73α, pSG5 HA-p73Δ493-521, or pSG5 HA-p73β, and 24-h post transfection, cells were cultured without (-) or with (+) 300 μM dicoumarol for 5 h. (H) HCT116 cells were cultured without (-) or with (+) 200 μM dicoumarol and without (-) or with (+) 50 μM lactacystin for 5 h. (I) A31N-ts20 cells were transiently transfected with pSG5 HA-p73α, incubated for 24 h at the restrictive temperature (39°C), and then cultured for 5 h without (-) or with (+) 300 μM dicoumarol. Immunoblot analysis (IB) was carried out with the following antibodies: mouse anti-Flag, mouse anti-HA, goat anti-NQO1, rabbit anti-p73, mouse anti-mouse p53, mouse anti-Actin, and rabbit anti-C9 to identify the 20S proteasomes. 35S-labeled p73 and NQO1 were detected by autoradiography.
Figure 5.
NQO1 stabilizes p73α and p53 following γ-irradiation. (A) 293 HEK cells were transiently transfected with pRc/CMV Flag-p53 without (-) or with (+) pEFIRES NQO1. Twenty-four hours following transfection, cells were γ-irradiated at 4 Gy (IR) and cultured for an additional 4 h (TOTAL). Flag-p53 was immunoprecipitated with anti-Flag beads (IP: Flag). The beads were washed without (-) or with (+) 300 μM dicoumarol (dicoumarol wash). (B) 293 HEK cells were transiently transfected with pEFIRES Flag-p73β or pEFIRES Flag-p73α, together with pEFIRES NQO1. Twenty-four hours following transfection, cells were γ-irradiated at 4 Gy (IR) and cultured for an additional 4 h (TOTAL). Flag-p73 was immunoprecipitated with anti-Flag beads (IP: Flag). (C) HCT116 cells were γ-irradiated at 6 Gy (IR) and cultured without (-) or with 200 or 400 μM dicoumarol for 4 h. (D) A31N-ts20 cells were incubated for 24 h at the permissive (32°C) or restrictive (39°C) temperature. Cells cultured at 39°C were then γ-irradiated at 4 Gy (IR) and cultured for additional 4 h at 39°C. (E) A31N-ts20 cells were transiently transfected with pRc/CMV human p53[22,23] without or with pSUPER NQO1 encoding NQO1-specific siRNA. Cells were then incubated for 24 h at 39°C, γ-irradiated at 4 Gy (IR), and cultured for an additional 4 h at 39°C. (F) A schematic model of p53 accumulation following IR. Protein extraction and immunoblot analysis (IB) were carried out as described in the Materials and Methods with mouse anti-mouse p53, mouse anti-human p53, mouse anti-Flag antibody, goat anti-NQO1 antibody, rabbit anti-p73, and mouse anti-Actin or anti-Tubulin antibodies.
Similar articles
- Mdm-2 and ubiquitin-independent p53 proteasomal degradation regulated by NQO1.
Asher G, Lotem J, Sachs L, Kahana C, Shaul Y. Asher G, et al. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):13125-30. doi: 10.1073/pnas.202480499. Epub 2002 Sep 13. Proc Natl Acad Sci U S A. 2002. PMID: 12232053 Free PMC article. - JNK-NQO1 axis drives TAp73-mediated tumor suppression upon oxidative and proteasomal stress.
Kostecka A, Sznarkowska A, Meller K, Acedo P, Shi Y, Mohammad Sakil HA, Kawiak A, Lion M, Królicka A, Wilhelm M, Inga A, Zawacka-Pankau J. Kostecka A, et al. Cell Death Dis. 2014 Oct 23;5(10):e1484. doi: 10.1038/cddis.2014.408. Cell Death Dis. 2014. PMID: 25341038 Free PMC article. - p53 proteasomal degradation: poly-ubiquitination is not the whole story.
Asher G, Shaul Y. Asher G, et al. Cell Cycle. 2005 Aug;4(8):1015-8. doi: 10.4161/cc.4.8.1900. Epub 2005 Aug 7. Cell Cycle. 2005. PMID: 16082197 Review. - Ubiquitin-independent p53 proteasomal degradation.
Tsvetkov P, Reuven N, Shaul Y. Tsvetkov P, et al. Cell Death Differ. 2010 Jan;17(1):103-8. doi: 10.1038/cdd.2009.67. Cell Death Differ. 2010. PMID: 19557012 Review.
Cited by
- NOXA, a sensor of proteasome integrity, is degraded by 26S proteasomes by an ubiquitin-independent pathway that is blocked by MCL-1.
Craxton A, Butterworth M, Harper N, Fairall L, Schwabe J, Ciechanover A, Cohen GM. Craxton A, et al. Cell Death Differ. 2012 Sep;19(9):1424-34. doi: 10.1038/cdd.2012.16. Epub 2012 Feb 24. Cell Death Differ. 2012. PMID: 22361683 Free PMC article. - Pathogenesis of colorectal carcinoma and therapeutic implications: the roles of the ubiquitin-proteasome system and Cox-2.
Voutsadakis IA. Voutsadakis IA. J Cell Mol Med. 2007 Mar-Apr;11(2):252-85. doi: 10.1111/j.1582-4934.2007.00032.x. J Cell Mol Med. 2007. PMID: 17488476 Free PMC article. Review. - Ubiquitin-dependent and ubiquitin-independent control of subunit stoichiometry in the SWI/SNF complex.
Keppler BR, Archer TK. Keppler BR, et al. J Biol Chem. 2010 Nov 12;285(46):35665-74. doi: 10.1074/jbc.M110.173997. Epub 2010 Sep 9. J Biol Chem. 2010. PMID: 20829358 Free PMC article. - p73 Alternative Splicing: Exploring a Biological Role for the C-Terminal Isoforms.
Vikhreva P, Melino G, Amelio I. Vikhreva P, et al. J Mol Biol. 2018 Jun 22;430(13):1829-1838. doi: 10.1016/j.jmb.2018.04.034. Epub 2018 May 4. J Mol Biol. 2018. PMID: 29733853 Free PMC article. Review. - Acetylation and phosphorylation of SRSF2 control cell fate decision in response to cisplatin.
Edmond V, Moysan E, Khochbin S, Matthias P, Brambilla C, Brambilla E, Gazzeri S, Eymin B. Edmond V, et al. EMBO J. 2011 Feb 2;30(3):510-23. doi: 10.1038/emboj.2010.333. Epub 2010 Dec 14. EMBO J. 2011. PMID: 21157427 Free PMC article.
References
- Anwar A., Dehn, D., Siegel, D., Kepa, J.K., Tang, L.J., Pietenpol, J.A., and Ross, D. 2003. Interaction of human NAD(P)H:quinone oxidoreductase 1 (NQO1) with the tumor suppressor protein p53 in cells and cell-free systems. J. Biol. Chem. 278: 10368-10373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous