PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature - PubMed (original) (raw)
PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature
Taija Mäkinen et al. Genes Dev. 2005.
Erratum in
- Genes Dev. 2006 Jul 1;20(13):1829
Abstract
The transmembrane ligand ephrinB2 and its cognate Eph receptor tyrosine kinases are important regulators of embryonic blood vascular morphogenesis. However, the molecular mechanisms required for ephrinB2 transduced cellular signaling in vivo have not been characterized. To address this question, we generated two sets of knock-in mice: ephrinB2DeltaV mice expressed ephrinB2 lacking the C-terminal PDZ interaction site, and ephrinB2(5F) mice expressed ephrinB2 in which the five conserved tyrosine residues were replaced by phenylalanine to disrupt phosphotyrosine-dependent signaling events. Our analysis revealed that the homozygous mutant mice survived the requirement of ephrinB2 in embryonic blood vascular remodeling. However, ephrinB2DeltaV/DeltaV mice exhibited major lymphatic defects, including a failure to remodel their primary lymphatic capillary plexus into a hierarchical vessel network, hyperplasia, and lack of luminal valve formation. Unexpectedly, ephrinB2(5F/5F) mice displayed only a mild lymphatic phenotype. Our studies define ephrinB2 as an essential regulator of lymphatic development and indicate that interactions with PDZ domain effectors are required to mediate its functions.
Figures
Figure 1.
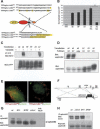
Generation and validation of novel ephrinB2 cDNA knock-in mutants. (A) Schematic representation of YFP-ephrinB2 mutants with the relevant amino acid sequence alterations in their cytoplasmic tails. (Top) EphrinB2ΔV and ephrinB25F compared with wild-type ephrinB2. (Bottom) Previously reported alleles of ephrinB2 including a substitution of HA-tag for the cytoplasmic domain (ephrinB2ΔC-HA; Adams et al. 1999) and a substitution of the ORF encoded by a loxP site for the cytoplasmic domain (ephrinB2ΔC-lox; Dravis et al. 2004). (B) Quantitative analysis of surface ephrinB2 in HeLa cells transiently transfected with GFP (control, _left_-most lane) or ephrinB2 wild-type or mutant constructs, as assayed by binding of EphB4-alkaline phosphatase fusion protein. Results of a representative experiment are presented as the average of bound alkaline phosphatase activity divided by total expressed protein and normalized to values for the wild-type construct (see Materials and Methods). Transfection of cells with wild-type (WT), 5F, ΔV, or ΔC-HA ephrinB2 expression plasmids result in robust binding of EphB4-AP, whereas ΔC-lox transfected cells exhibit little surface-binding activity, consistent with the protein product being trapped in the trans-Golgi network (Cowan et al. 2004). ★★ indicates p < 0.01 for triplicate measurements, Student's two-tailed _t_-test. (C) EphrinB2 tyrosine phosphorylation after vanadate treatment. Hela cells transiently transfected with wild-type (WT), tyrosine mutant (5F), or PDZ site-deficient (ΔV) ephrinB2 constructs were stimulated with vanadate and lysed. Immunoprecipitated ephrinB2 was analyzed by anti-phosphotyrosine (4G10) and total ephrinB2 by anti-YFP Western blot. (D) Interaction of ephrinB2 with PDZ proteins. HeLa cells transiently transfected with wild-type (WT) or PDZ-binding site-deficient (ΔV, Δ4) ephrinB2 constructs were lysed and the lysates subjected to pull-down using bacterially produced syntenin1 fused to maltose-binding protein (SY) or unfused maltose-binding protein (MBP). (Top) The resultant pull-downs were analyzed by anti-ephrinB2 Western blot. (Bottom) Pull-down input was analyzed by Western blot against ephrinB2 on total lysates. (E) Colocalization of PDZ domain containing protein, syntenin, with ephrinB2. Hela cells transiently transfected with wild-type (WT) or PDZ-binding site-deficient (ΔV) YFP-ephrinB2 constructs. Cells were stimulated for 30 min with clustered EphB4-Fc receptor bodies to induce ephrinB2 clustering (Zimmer et al. 2003). A mutant GFP-tagged syntenin, lacking PIP2-binding activity, colocalized robustly with patches of ephrinB2WT (left panel) but not with ephrinB2ΔV (right panel). Bar, 10 μm. (F) Targeting strategy for the generation of mutant mice expressing ephrinB25F or ephrinB2_Δ_V. Exon 1 (gray box) contains the 5′ end of the ORF. The depicted cDNA knock-in strategy was described previously (Adams et al. 2001; see also Materials and Methods). The loxP-flanked (black triangles) neomycin selection marker (black box) was subsequently removed by intercrossing with a mouse line transgenic for Cre recombinase. HindIII restriction sites (“H”) and the probe used for Southern hybridization are indicated. (G) EphrinB2 expression levels in adult brain lysates. EphrinB2 was immunoprecipitated from total adult brain lysates of wild-type and heterozygous (5F/+), homozygous (5F/5F and ΔV/ΔV) knock-in mice, or ephrinB2lx/lx;Nestin-Cre mutants (lx/lx;NCre) (Grunwald et al. 2004). The resulting precipitates were analyzed by anti-ephrinB2 Western blot. Mutant ephrinB2 is translated to approximately native levels. (H) EphrinB2 tyrosine phosphorylation in E12.5 embryos. EphrinB2 was immunoprecipitated as in G from E12.5 wild-type and homozygous mutant animals and the precipitates analyzed for phosphotyrosine content by anti-phosphotyrosine (4G10) Western blot. Total ephrinB2 was analyzed by anti-ephrinB2 Western blot.
Figure 2.
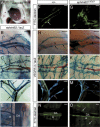
Collecting lymphatic vessels are hyperplastic and unvalved in ephrinB2_Δ_V/Δ_V_ mice. (A) Chylothorax in an ephrinB2_Δ_V/Δ_V_ mutant mouse. Gross dissection of the chest cavity of a P6 ephrinB2_Δ_V/Δ_V_ mutant cadaver showing chest cavity filled with chyle (★). (h) Heart. (_B_-E) X-Gal staining in heterozygous ephrinB2LZ/+ reporter mice. In addition to strong expression in the arterial endothelium and SMCs, ephrinB2 promoter activity is detected in the endothelium of collecting lymphatic vessels surrounding the ischiatic vein (B), in the skin (C), mesentery (D), and thoracic duct (white arrowhead, E). (B_-D) While venous endothelium (V) is negative for ephrinB2 expression, the SMCs surrounding larger veins are positive (see also Supplementary Fig. S2). Luminal valves are indicated with black arrows. (L) Lymphatic vessel; (A) artery; (V) vein; (DA) dorsal aorta. (F_-O) Fluorescent lymphoscintiography of the collecting lymphatic vessels surrounding the ischiatic vein (F,G) and of the thoracic duct (N,O) after high-molecular-weight FITC-dextran injection into the hindlimb footpad of wild-type (F,N) and ephrinB2_Δ_V/Δ_V mutant (G,O) mice. X-Gal staining of lymphatic vessels in VEGFR3LZ/+ mice of ischiatic vein region (H,I), collecting lymphatic vessels of the skin (J,K), and mesenteric lymphatic vessels (L,M) of the indicated genotypes (top). The collecting lymphatic vessels are hyperplastic in the mutant mice (G,I,K) and lack the luminal valves present in wild-type mice (arrows in panels F,H,J,L). The thoracic duct is indicated by white arrowheads in panels N and O; the leakage and reflux of FITC dextran dye into the side branches in ephrinB2_Δ_V/Δ_V mutant mouse is indicated by white arrows in O. Bars: _B_-_D,F_-M, 200 μm; E,N,O, 500 μm. Ages of the mice: _B,F_-I,N,O, P10; D,E,L,M, P5; C,J,K, P1.
Figure 3.
Defective remodeling of the lymphatic capillary plexus in ephrinB2_Δ_V/Δ_V_ mutant skin. Whole-mount X-gal staining in the VEGFR3LZ/+ background of wild-type (+/+; A,E,e_′,I,M) and ephrinB2_Δ_V/Δ_V (_B,F,f_′,J,N) mutant skin (respective sections in panels C,D,K,L). Ventral skin biopsies were taken from mice at the indicated ages (P0-P5). Sprouts forming from the primary plexus in wild-type skin are indicated by filled arrowheads in panel E (higher magnification in _e_′), secondary and tertiary sprouts by a black arrow in panel I. (K,M,O) Sprouts elongate to upper dermal layers and form a superficial lymphatic capillary plexus (arrows). In the mutants, the sprouting is disturbed (open arrowheads in F, higher magnification in _f_′), which leads to failure in the formation of the superficial capillary plexus (J,L,N). (G,O) Combined X-Gal and anti-VEGFR-3 staining in heterozygous ephrinB2LZ/+ skin at P1 (G) and at P5 (O). Note double staining of primary lymphatic capillary plexus and smaller diameter precollectors (arrowheads in G,O, respectively) for ephrinB2 (X-Gal; blue) and VEGFR-3 (IHC; red). (O) The post-remodeling superficial plexus is ephrinB2 negative (arrows). (G,O) In addition to endothelial cells, ephrinB2 is also expressed in hair follicles and upper layers of the dermis. (H) X-Gal staining of EphB4LZ/+ skin at P1 revealed EphB4 expression in primary lymphatic capillaries (arrowhead) in addition to veins. Bars, left panels and H (whole mounts), 200 μm; right panels (sections), 50 μm. (_e_′,_f_′) Higher magnifications from E and F.
Figure 6.
EphrinB2 is expressed predominantly in the endothelial cells of collecting lymphatic vessels, while EphB4 is in both lymphatic capillaries and collecting lymphatic vessels. Triple immunofluorescence for LYVE-1, podoplanin, and αSMA (_A_-D); for ephrinB2 (βgal), podoplanin, and αSMA (_E_-H); and for EphB4, podoplanin, and LYVE-1 (_I_-L) of the ear skin at P21. (_A_-D) High LYVE-1 expression is detected in the lymphatic capillaries (arrow) but not in the αSMA-positive collecting lymphatic vessels (arrowhead). Podoplanin instead stains all lymphatic vessels. αSMA also stains blood vessels (bv). (_E_-H) EphrinB2 is expressed on the endothelium of collecting lymphatic vessels (αSMA-positive, arrowhead), but not on podoplanin-positive, αSMA-negative lymphatic capillaries (arrow). (_I_-L) EphB4 is expressed both on the LYVE-1-positive lymphatic capillaries (arrow) and on the LYVE-1-negative collecting lymphatic vessels (arrowhead). In addition, the veins are positive for EphB4. Bars, 100 μm.
Figure 4.
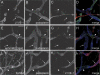
Abnormal lymphatic drainage in ephrinB2 mutant mice. (_A_-E) Visualization of the dermal lymphatic vessels using fluorescence microscopy after FITC-dextran injection into the forelimb footpad of wild-type mice (+/+) at P0 (A) and at P10 (B), and of ephrinB2 knock-in mutants at P10 (C_-E). (A) At P0, the whole primitive dermal lymphatic vessel network is visualized after FITC-dextran injection. At P10, in wild-type (B) and knock-in control (C) mouse skin, only subcutaneous collecting lymphatic vessels (arrowheads) are visualized, while in ephrinB2_Δ_V/Δ_V (D) and ephrinB25F/5F (E) mutant mice a vessel network is observed. Bars, 500 μm.
Figure 5.
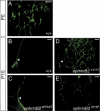
Defective remodeling and abnormal SMC coverage of lymphatic capillaries in ephrinB2_Δ_V/Δ_V_ mice. (A_-F) Immunofluorescence using antibodies against lymphatic-specific marker LYVE-1 of the ear skin at P3 and P5. The formation of the primary lymphatic capillary plexus and the formation of filopodia occurred normally in ephrinB2_Δ_V/Δ_V mice (B,D) when compared with wild-type controls (A,C), but the elongation of the sprouting tip cell (arrowhead in E) failed in the mutants, resulting in blunt-ended protrusions (arrowhead in F). (G,H) Double immunofluorescence using antibodies against LYVE-1 (green) and podoplanin (red) of the ear skin at P21. (G) Note that in wild-type mice LYVE-1 is expressed in the endothelial cells of lymphatic capillaries (arrow), while podoplanin is expressed both in the capillaries and valved collecting lymphatic vessels (arrowhead). (H) In the ephrinB2_Δ_V/Δ_V_ mice the whole hyperplastic lymphatic vessel network is LYVE-1 positive. (_I_-M) Double immunofluorescence using antibodies against α-smooth muscle actin (αSMA) for SMCs (red) and against LYVE-1 (green) of the ear skin of the indicated genotypes and ages. (I_-K) While blood vessels stain positive for αSMA (open arrowheads), the lymphatic capillary network (green) of wild-type and ephrinB25F/5F mice is devoid of αSMA staining both at P5 and P21. (J) However, the hyperplastic lymphatic capillaries of ephrinB2_Δ_V/Δ_V mice at P21 stain positive for αSMA (white arrow pointing to reddish yellow staining). (a) Artery; (v) vein. Bars, _A_-F, 50 μm; _G_-M, 100 μm.
Figure 7.
Abnormal localization of ephrinB2 interactors PDZ-RGS3 and Dvl2 in the lymphatic endothelia of ephrinB2_Δ_V/Δ_V_ mice. (_A_-C) Double immunofluorescence staining of the ear skin at P21 for ephrinB2 (A; green channel in C) and for PECAM-1 (B; red channel in C). (A,C) Note that ephrinB2 is localized in spots in a region of a lymphatic valve (arrowheads). (D_-I) Double immunofluorescence staining of the ear skin of wild-type (D,F,H) and ephrinB2_Δ_V/Δ_V (E,G,I) mice at P21 for PICK1 (green channel in D,E; left part shows PICK1 channel only in b/w) and PDZ-RGS3 (green channel in F,G) together with podoplanin (blue channel in D_-G). (H,I) Double immunofluorescence staining for Dvl2 (green) with PECAM-1 (red). (F,H) Similar to the localization of ephrinB2, several of the interactors form spots in the lymphatic endothelia in wild-type skin, especially at the valve regions (arrowheads). The localization of PICK1 is not disturbed in ephrinB2_Δ_V/Δ_V mice (D,E), while PDZ-RGS3 shows a diffuse staining (arrow in G) and Dvl2 is undetectable in the mutant lymphatic vessels, even at the sites where abnormal valve-like structures have formed (arrow in I). Bars, 50 μm.
Similar articles
- EphrinB2-EphB4 signalling provides Rho-mediated homeostatic control of lymphatic endothelial cell junction integrity.
Frye M, Stritt S, Ortsäter H, Hernandez Vasquez M, Kaakinen M, Vicente A, Wiseman J, Eklund L, Martínez-Torrecuadrada JL, Vestweber D, Mäkinen T. Frye M, et al. Elife. 2020 Sep 8;9:e57732. doi: 10.7554/eLife.57732. Elife. 2020. PMID: 32897857 Free PMC article. - EphB4 forward signalling regulates lymphatic valve development.
Zhang G, Brady J, Liang WC, Wu Y, Henkemeyer M, Yan M. Zhang G, et al. Nat Commun. 2015 Apr 13;6:6625. doi: 10.1038/ncomms7625. Nat Commun. 2015. PMID: 25865237 Free PMC article. - EphrinB2-EphB4 signals regulate formation and maintenance of funnel-shaped valves in corneal lymphatic capillaries.
Katsuta H, Fukushima Y, Maruyama K, Hirashima M, Nishida K, Nishikawa S, Uemura A. Katsuta H, et al. Invest Ophthalmol Vis Sci. 2013 Jun 12;54(6):4102-8. doi: 10.1167/iovs.12-11436. Invest Ophthalmol Vis Sci. 2013. PMID: 23696610 - Regulation of signaling interactions and receptor endocytosis in growing blood vessels.
Pitulescu ME, Adams RH. Pitulescu ME, et al. Cell Adh Migr. 2014;8(4):366-77. doi: 10.4161/19336918.2014.970010. Cell Adh Migr. 2014. PMID: 25482636 Free PMC article. Review. - EphrinB2-EphB4 Signaling in Neurooncological Disease.
Piffko A, Uhl C, Vajkoczy P, Czabanka M, Broggini T. Piffko A, et al. Int J Mol Sci. 2022 Jan 31;23(3):1679. doi: 10.3390/ijms23031679. Int J Mol Sci. 2022. PMID: 35163601 Free PMC article. Review.
Cited by
- Lymph flow regulates collecting lymphatic vessel maturation in vivo.
Sweet DT, Jiménez JM, Chang J, Hess PR, Mericko-Ishizuka P, Fu J, Xia L, Davies PF, Kahn ML. Sweet DT, et al. J Clin Invest. 2015 Aug 3;125(8):2995-3007. doi: 10.1172/JCI79386. Epub 2015 Jul 27. J Clin Invest. 2015. PMID: 26214523 Free PMC article. - Neural Regulation of Vascular Development: Molecular Mechanisms and Interactions.
Zhang Y, Shen X, Deng S, Chen Q, Xu B. Zhang Y, et al. Biomolecules. 2024 Aug 8;14(8):966. doi: 10.3390/biom14080966. Biomolecules. 2024. PMID: 39199354 Free PMC article. Review. - Smooth muscle-endothelial cell communication activates Reelin signaling and regulates lymphatic vessel formation.
Lutter S, Xie S, Tatin F, Makinen T. Lutter S, et al. J Cell Biol. 2012 Jun 11;197(6):837-49. doi: 10.1083/jcb.201110132. Epub 2012 Jun 4. J Cell Biol. 2012. PMID: 22665518 Free PMC article. - Eph-dependent cell-cell adhesion and segregation in development and cancer.
Nievergall E, Lackmann M, Janes PW. Nievergall E, et al. Cell Mol Life Sci. 2012 Jun;69(11):1813-42. doi: 10.1007/s00018-011-0900-6. Epub 2011 Dec 28. Cell Mol Life Sci. 2012. PMID: 22204021 Free PMC article. Review. - Lymphatic development.
Butler MG, Isogai S, Weinstein BM. Butler MG, et al. Birth Defects Res C Embryo Today. 2009 Sep;87(3):222-31. doi: 10.1002/bdrc.20155. Birth Defects Res C Embryo Today. 2009. PMID: 19750516 Free PMC article. Review.
References
- Adams R.H. 2002. Vascular patterning by Eph receptor tyrosine kinases and ephrins. Semin. Cell Dev. Biol. 13: 55-60. - PubMed
- Adams R.H., Wilkinson, G.A., Weiss, C., Diella, F., Gale, N.W., Deutsch, U., Risau, W., and Klein, R. 1999. Roles of ephrinB ligands and EphB receptors in cardiovascular development: De-marcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes & Dev. 13: 295-306. - PMC - PubMed
- Adams R.H., Diella, F., Hennig, S., Helmbacher, F., Deutsch, U., and Klein, R. 2001. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell 104: 57-69. - PubMed
- Alitalo K. and Carmeliet, P. 2002. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 1: 219-227. - PubMed
- Brambilla R., Bruckner, K., Orioli, D., Bergemann, A.D., Flanagan, J.G., and Klein, R. 1996. Similarities and differences in the way transmembrane-type ligands interact with the Elk subclass of Eph receptors. Mol. Cell. Neurosci. 8: 199-209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous