Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex - PubMed (original) (raw)
Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex
Victoria Y Gorbacheva et al. Proc Natl Acad Sci U S A. 2005.
Abstract
The circadian clock controls many aspects of mammalian physiology, including responses to cancer therapy. We find that wild-type and circadian mutant mice demonstrate striking differences in their response to the anticancer drug cyclophosphamide (CY). While the sensitivity of wild-type mice varies greatly, depending on the time of drug administration, Clock mutant and Bmal1 knockout mice are highly sensitive to treatment at all times tested. On the contrary, mice with loss-of-function mutations in Cryptochrome (Cry1-/-Cry2-/- double knockouts) were more resistant to CY compared with their wild-type littermates. Thus, both time-of-day and allelic-dependent variations in response to chemotherapy correlate with the functional status of the circadian CLOCK/BMAL1 transactivation complex. Pharmacokinetic analysis of plasma concentration of different CY metabolites shows that, in contrast to the traditional view, circadian variations in drug sensitivity cannot be attributed to the changes in the rates of CY metabolic activation and/or detoxification. At the same time, mice of different circadian genotypes demonstrate significant differences in B cell responses to toxic CY metabolites: B cell survival/recovery rate was directly correlated with the in vivo drug sensitivity. Based on these results, we propose that the CLOCK/BMAL1 transcriptional complex affects the lethality of chemotherapeutic agents by modulating the survival of the target cells necessary for the viability of the organism.
Figures
Fig. 1.
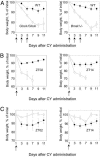
Effect of time of treatment on in vivo drug response in wild-type C57BL/6J mice. (A) Survival of C57BL/6J mice after 3 × 150 mg/kg i.p. injections of CY performed at different times of the day. Animals injected at ZT10 to ZT14 demonstrate higher survival rate compared with their littermates injected at ZT22 to ZT02. (B) Dose–response curve for the survival rate of C57BL/6J mice injected at ZT14 (•) or ZT02 (○). Animals injected at ZT14 can better tolerate higher doses of the drug. (C) Total body weight loss in C57BL/6J mice after 3 × 100 mg/kg CY i.p. injections administered at ZT14 (•) or ZT02 (○). (D) Results are the same as in C after 3 × 200 mg/kg i.p. injections. At both doses, percent of body weight loss is significantly higher if injections were performed at ZT02 (at day 9, P = 0.002). Arrows indicate the days of treatment. Values represent mean ± SEM.
Fig. 2.
Effect of circadian mutations on in vivo drug response. (A) Body weight loss of control (•), Clock/Clock (Left, ○), and _Bmal1_-/- knockout mice (Right, ○). Ten animals of each genotype received 3 × 150 mg/kg CY at ZT10, the time of the highest resistance for control group. Both mutants respond to CY treatment by significantly higher total body weight loss as compared with their wild-type littermates. (B) Body weight loss of control (•) and Clock/Clock mutant (○) mice after a single 300 mg/kg CY injection performed at ZT02 (Left) or ZT14 (Right). At both times used, Clock/Clock mice demonstrate significantly higher drug sensitivity. (C) Body weight loss of control (•) and _Cry1_-/- _Cry2_-/- (○) knockout mice after a single 300 mg/kg CY dose administered at ZT02 (Left) or ZT14 (Right). _Cry1_-/-_Cry2_-/- double-knockout mice are more resistant to the treatment than their wild-type littermates (at day 9, P = 0.07 for ZT02 and P = 0.02 for ZT14).
Fig. 3.
PK analysis of CY metabolites in the plasma of wild-type and Clock mutant mice. (A) Partial metabolic scheme for CY activation and detoxification. The initial activation step is mediated by the cytochrome P450 enzymes through two alternative pathways: the first pathway, leading to formation of 4-OH, and the second pathway, yielding biologically inactive DCE. 4-OH is a circulating metabolite whose spontaneous decomposition by means of aldophosphoamide yields the ultimate alkylating metabolite, PM, and acrolein. Alternatively, 4-OH may be enzymatically detoxified, yielding several inactive compounds. Cyp3a13, Cyp2b10, and Cyp2c29 are cytochrome P450 isoforms involved in the initial CY metabolic transformation. Aldh, aldehyde dehydrogenase. (B) Concentration versus time curves of CY and its toxic metabolites, 4-OH and PM, in the plasma of wild-type mice injected at ZT14 (black circles) and ZT02 (white circles) and Clock/Clock mice (gray circles). (Insets) Corresponding semilog plots used to calculate the _t_1/2 values for ZT02, ZT14, and Clock/Clock groups (CY: 11.85 ± 0.26, 12.74 ± 1.5, and 13.75 ± 1.77 min; 4-OH: 15.8 ± 5.4, 13.2 ± 1.6, and 13.9 ± 0.5 min; PM: 35.8 ± 8.4, 29.5 ± 7.8, and 28.45 ± 4.9 min). (C) Results are for the same curves for the inactive metabolites, DCE and CB. Three PK experiments performed with different dosing and administration schedule gave similar results. (D) Real-time PCR assay for Cyp3a13 mRNA abundance in the liver of control and Clock/Clock mice. Wild-type and Clock/Clock mice were entrained to a 12-h light:12-h dark cycle for 1 week before tissue collection. Three animals of each genotype were killed at ZT02, ZT06, ZT10, ZT14, ZT18, and ZT22; livers were removed, frozen on dry ice, and stored at -80°C until RNA isolation. Values on the bar graphs presented combined average values for all 18 animals of each genotype ± SEM.
Fig. 4.
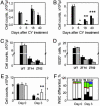
The functional status of CLOCK/BMAL1 transcriptional complex modulates the sensitivity of hematopoietic cells to CY-induced toxicity. (A) Neutrophil counts in peripheral blood of wild-type (black bars) and Clock/Clock mice (gray bars) measured at different times after 3 × 150 mg/kg CY injected at ZT14. At day 5 after treatment, the amount of neutrophils in Clock/Clock mice is slightly lower (P = 0.1). (B) Lymphocyte counts in the same blood samples. At all times tested, Clock mutant mice show significant lower number of circulating lymphocytes (*, P = 0.02; **, P = 0.004; ***, P = 0.01) when compared with wild-type controls. (C) Lymphocyte counts in peripheral blood of wild-type and Clock/Clock mice at day 3 after 1 × 300 mg/kg CY injected at ZT14 or ZT02. A generalized linear model ANOVA detects significant effect of genotype (df = 1, F = 20.83, P = 0.000115) and time of injection (df = 1, F = 4.28, P = 0.049). No significant interaction of two parameters was detected. (D) B220-positive cells in the bone marrow of wild-type and Clock/Clock mice at day 3 after 1 × 300 mg/kg CY injection at ZT14 and ZT02. A generalized linear model ANOVA detects significant effect of genotype (df = 1, F = 31.55, P = 0.000039) and time of injection (df = 1, F = 24.37, P = 0.000149). No significant interaction was detected. (E) Lymphocyte counts in peripheral blood of mice of different circadian genotypes after 1 × 300 mg/kg CY administration at ZT11.5. No differences detected in untreated mice of all three genotypes. However, at day 3, Clock/Clock mice show significantly more severe reduction in lymphocyte survival rate compared with wild-type animals (*, P = 0.03), whereas _Cry1_-/-_Cry2_-/- mice retain higher lymphocyte levels (**, P = 0.05). (F) WBC composition of wild-type, Clock/Clock, and _Cry1_-/-_Cry2_-/- mice at days 0 and 3 after 1 × 300 mg/kg CY administration. Each stack in the bar represents cell type percentage: neutrophils are black, lymphocytes are white, monocytes are red, eosinophils are green, basophils are yellow, and the large unstained cell population is blue. W, wild-type; Cl, Clock/Clock; Cr, _Cry1_-/-_Cry2_-/-.
Comment in
- Time for chronotherapy? Clock genes dictate sensitivity to cyclophosphamide.
Green CB. Green CB. Proc Natl Acad Sci U S A. 2005 Mar 8;102(10):3529-30. doi: 10.1073/pnas.0500552102. Epub 2005 Feb 28. Proc Natl Acad Sci U S A. 2005. PMID: 15738390 Free PMC article. No abstract available.
Similar articles
- Dual role of the CLOCK/BMAL1 circadian complex in transcriptional regulation.
Kondratov RV, Shamanna RK, Kondratova AA, Gorbacheva VY, Antoch MP. Kondratov RV, et al. FASEB J. 2006 Mar;20(3):530-2. doi: 10.1096/fj.05-5321fje. Epub 2006 Jan 25. FASEB J. 2006. PMID: 16507766 - BMAL1-dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system.
Kondratov RV, Chernov MV, Kondratova AA, Gorbacheva VY, Gudkov AV, Antoch MP. Kondratov RV, et al. Genes Dev. 2003 Aug 1;17(15):1921-32. doi: 10.1101/gad.1099503. Genes Dev. 2003. PMID: 12897057 Free PMC article. - Preferential inhibition of BMAL2-CLOCK activity by PER2 reemphasizes its negative role and a positive role of BMAL2 in the circadian transcription.
Sasaki M, Yoshitane H, Du NH, Okano T, Fukada Y. Sasaki M, et al. J Biol Chem. 2009 Sep 11;284(37):25149-59. doi: 10.1074/jbc.M109.040758. Epub 2009 Jul 15. J Biol Chem. 2009. PMID: 19605937 Free PMC article. - Circadian clock genes as modulators of sensitivity to genotoxic stress.
Antoch MP, Kondratov RV, Takahashi JS. Antoch MP, et al. Cell Cycle. 2005 Jul;4(7):901-7. doi: 10.4161/cc.4.7.1792. Epub 2005 Jul 26. Cell Cycle. 2005. PMID: 15917646 Free PMC article. Review. - Genetics and neurobiology of circadian clocks in mammals.
Siepka SM, Yoo SH, Park J, Lee C, Takahashi JS. Siepka SM, et al. Cold Spring Harb Symp Quant Biol. 2007;72:251-259. doi: 10.1101/sqb.2007.72.052. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419282 Free PMC article. Review.
Cited by
- Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling.
Plikus MV, Vollmers C, de la Cruz D, Chaix A, Ramos R, Panda S, Chuong CM. Plikus MV, et al. Proc Natl Acad Sci U S A. 2013 Jun 4;110(23):E2106-15. doi: 10.1073/pnas.1215935110. Epub 2013 May 20. Proc Natl Acad Sci U S A. 2013. PMID: 23690597 Free PMC article. - Emerging links between the biological clock and the DNA damage response.
Collis SJ, Boulton SJ. Collis SJ, et al. Chromosoma. 2007 Aug;116(4):331-9. doi: 10.1007/s00412-007-0108-6. Epub 2007 May 11. Chromosoma. 2007. PMID: 17492458 Review. - Foundations of circadian medicine.
Kramer A, Lange T, Spies C, Finger AM, Berg D, Oster H. Kramer A, et al. PLoS Biol. 2022 Mar 24;20(3):e3001567. doi: 10.1371/journal.pbio.3001567. eCollection 2022 Mar. PLoS Biol. 2022. PMID: 35324893 Free PMC article. - Daily Time of Radiation Treatment Is Associated with Subsequent Oral Mucositis Severity during Radiotherapy in Head and Neck Cancer Patients.
Gu F, Farrugia MK, Duncan WD, Feng Y, Hutson AD, Schlecht NF, Repasky EA, Antoch MP, Miller A, Platek A, Platek ME, Iovoli AJ, Singh AK. Gu F, et al. Cancer Epidemiol Biomarkers Prev. 2020 May;29(5):949-955. doi: 10.1158/1055-9965.EPI-19-0961. Epub 2020 Feb 25. Cancer Epidemiol Biomarkers Prev. 2020. PMID: 32098893 Free PMC article. - Time for chronotherapy? Clock genes dictate sensitivity to cyclophosphamide.
Green CB. Green CB. Proc Natl Acad Sci U S A. 2005 Mar 8;102(10):3529-30. doi: 10.1073/pnas.0500552102. Epub 2005 Feb 28. Proc Natl Acad Sci U S A. 2005. PMID: 15738390 Free PMC article. No abstract available.
References
- Reppert, S. M. & Weaver, D. R. (2002) Nature 418, 935-941. - PubMed
- Panda, S., Hogenesch, J. B. & Kay, S. A. (2002) Nature 417, 329-335. - PubMed
- Panda, S., Antoch, M. P., Miller, B. H., Su, A. I., Schook, A. B., Straume, M., Schultz, P. G., Kay, S. A., Takahashi, J. S. & Hogenesch, J. B. (2002) Cell 109, 307-320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA088071/CA/NCI NIH HHS/United States
- CA102522/CA/NCI NIH HHS/United States
- R01 CA102522/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- CA88071/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources