Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin - PubMed (original) (raw)
Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin
John G Lock et al. Mol Biol Cell. 2005 Apr.
Abstract
E-cadherin plays an essential role in cell polarity and cell-cell adhesion; however, the pathway for delivery of E-cadherin to the basolateral membrane of epithelial cells has not been fully characterized. We first traced the post-Golgi, exocytic transport of GFP-tagged E-cadherin (Ecad-GFP) in unpolarized cells. In live cells, Ecad-GFP was found to exit the Golgi complex in pleiomorphic tubulovesicular carriers, which, instead of moving directly to the cell surface, most frequently fused with an intermediate compartment, subsequently identified as a Rab11-positive recycling endosome. In MDCK cells, basolateral targeting of E-cadherin relies on a dileucine motif. Both E-cadherin and a targeting mutant, DeltaS1-E-cadherin, colocalized with Rab11 and fused with the recycling endosome before diverging to basolateral or apical membranes, respectively. In polarized and unpolarized cells, coexpression of Rab11 mutants disrupted the cell surface delivery of E-cadherin and caused its mistargeting to the apical membrane, whereas apical DeltaS1-E-cadherin was unaffected. We thus demonstrate a novel pathway for Rab11 dependent, dileucine-mediated, mu1B-independent sorting and basolateral trafficking, exemplified by E-cadherin. The recycling endosome is identified as an intermediate compartment for the post-Golgi trafficking and exocytosis of E-cadherin, with a potentially important role in establishing and maintaining cadherin-based adhesion.
Figures
Figure 1.
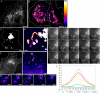
Expression of Ecad-GFP and transport in PGCs. HeLa cells were transiently transfected with Ecad-GFP (A–J) or VSVG-GFP (K and L). Ecad-GFP is concentrated in the perinuclear region 3 h after transfection (A) and is then visible at the PM in cell-cell contacts by 24 h (B). Shortly after transfection, newly synthesized Ecad-GFP (C) and the Golgi marker GM130 (D) colocalize in a merged image (E) in fixed HeLa cells. Ecad-GFP is visible in the Golgi region and in PGCs exiting the Golgi in a live HeLa cell (F). Regions from the image in F are magnified, digitally filtered and shown in relief to demonstrate two regions of interest containing Ecad-GFP–positive tubular PGCs (dashed box shown in G; dotted box shown in H). Arrows show tubular PGCs (5–20 μm; G and H), as they exit the Golgi region. Individual PGCs imaged after exit from the Golgi carrying either Ecad-GFP (I and J) or VSVG-GFP (K and L), from different transfected cells, include tubulovesicular PGCs (∼1 μm length) with concentrations of cargo at the trailing edge (I and K) or spherical carriers (250–300 nm diameter; J and L). Scale bars, (A–H) 10 μm; (I–L) 200 nm.
Figure 2.
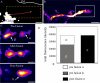
Ecad-GFP–positive PGC trajectories and direct Golgi-to-PM transport. Ecad-GFP was imaged in live HeLa cells to follow the trajectories of PGCs. A 60-s movie sequence (4 images/s) was recorded and then sequential images back-subtracted to produce a single image, showing the paths of seven Ecad-GFP–positive PGCs, with beginnings and ends of paths designated by yellow and red dots, respectively (A). A 90-s back-subtracted sequence, converted to an intensity-based color palette, highlights the concentration of curvilinear PGC tracks in the center of the cell (B). Imaging of a live HeLa cell at a focal plane level with the Golgi complex, shows the trajectory of a single spherical PGC (red trace) moving toward the cell periphery over a period of 40 s (C–E; Supplementary Movie 1). This is further enlarged in a back-subtracted image (D). Individual frames from this movie sequence (4 frames/s) show this 300-nm, spherical PGC (circled in red) as it exits the Golgi (frame 1) and finally disappears from the focal plane (after frame 111; E). To assess the fates of such PGCs in other live cells, imaging was performed at a focal plane level with the basal cell surface (F–H). Fusion events between Ecad-GFP-PGCs and the PM (F) are depicted in a region of interest (white box), converted to a pixel intensity-based color display palette (Supplementary Movie 2). Over 120 s, five fusion events were recorded, designated by white circles (G). Fully loaded PGCs arrived in the region, remained stationary, and then fused with the PM. In H, two stationary carriers (dashed box from G) are shown at 0.267-s intervals, fusing in succession. Analysis of the carrier fluorescence intensity distribution of the circled in H, is graphed I. The progressive flattening of the fluorescence distribution curve, indicates lateral dispersal of the PGC contents during fusion (Schmoranzer et al., 2000; Toomre et al., 2000). Scale bars, (A–C and F) 10 μm; (D, E, and G) 1 μm; (H) 250 nm.
Figure 3.
Ecad-GFP-PGCs fuse most frequently with intermediate compartments before PM delivery. Imaging in live HeLa cells shows the recorded track of an Ecad-GFP-PGC (red line; 56-s duration, 0.267 s/frame Supplementary Movie 3; A). The same trajectory is shown enlarged in a back-subtracted image (B) cropped from the dashed box in A. At the terminus of this trajectory (box from B) the PGC fuses with an intermediate, Ecad-GFP–positive structure, demonstrated by three sequential stages (C). Fluorescence intensities in the PGC and intermediate structure, objects β and α, respectively, in C, merge to give an equivalent total fluorescence in the final fused object, π in C, as graphed (D). Scale bars, (A and B) 2 μm; (C) 1 μm.
Figure 4.
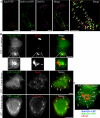
Biosynthetic E-cadherin colocalizes with Rab11wt-GFP in HeLa cells. HeLa cells cotransfected with Ecad-YFP and Rab11wt-GFP were fixed and labeled with an antibody to Golgi marker GM130 (A). Confocal microscopy and spectral unmixing show the individual patterns of each protein and the merged image. Ecad-YFP and Rab11wt-GFP colocalized in punctate structures (arrowheads) seen in the enlarged, merged image. Ecad-YFP also colocalized in some separate structures with GM130 (arrows). In B, HeLa cells coexpressing hEcad (red) and Rab11wt-GFP (green) were incubated at 20°C to block TGN exit and then fixed immediately (no release) or after 15- or 60-min release incubations at 37°C. An antibody used to label hEcad shows perinuclear Golgi accumulation, without PM labeling, and no colocalization with peripheral Rab11wt-GFP puncta, at 20°C. Enlargement of a typical perinuclear fluorescence pattern (dotted box) shows hEcad and Rab11wt-GFP closely adjacent, but not colocalizing. On release, hEcad then colocalizes in scattered punctate structures with Rab11wt-GFP (see solid arrows, 15 min, and hollow arrows, 60 min in merged images). hEcad labeling at the PM is evident 60 min after release. In HeLa cells expressing Rab11wt-YFP (green), hEcad (red), and Rab5QL-GFP (blue; C), hEcad colocalization with Rab11wt-YFP–positive punctae is clearly evident 15 min after 20°C block release (hollow arrows), although no significant colocalization is observed with Rab5QL-GFP–positive enlarged early endosomes (solid arrows). Scale bars, 10 μm.
Figure 5.
Colocalization of Ecad-mRFP with Rab11wt-GFP in live HeLa cells. HeLa cells, cotransfected with Rab11wt-GFP and Ecad-mRFP, were imaged live. A single image at the plane of the Golgi complex shows colocalization in some of the larger (1–3 μm), stationary structures (A). Ecad-mRFP is concentrated in the Golgi, in PGCs and in the larger structures colabeled with Rab11wt-GFP. Sequential images (cropped from dashed box in A) show colabeled intermediate compartments that move locally, whereas surrounding PGCs move on longer trajectories through this field (B). PGCs containing Rab11wt-GFP, Ecad-mRFP, or both, can be observed budding from and fusing with the intermediate compartments in the live movie sequence (Supplementary Movie 4). Scale bars, (A) 10 μm; (B) 5 μm.
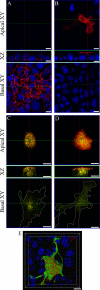
Figure 6.
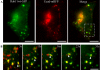
Localization and surface delivery of hEcad and ΔS1-Ecad in preconfluent HeLa and MDCK cells expressing Rab11 mutants. HeLa (A–C, E, and F) and MDCK (D) cells were fixed 24 h after transfection with combinations of either untagged hEcad or ΔS1-Ecad and either Rab11wt-GFP or Rab11QL-GFP. Cells were imaged by confocal microscopy as ZY/ZX/XY sections (A and E) or by epifluorescence (B–D and F). Immunolabeling of cells expressing either hEcad (A) or ΔS1-Ecad (E) alone, reveals efficient surface delivery of both cargos to the whole surface of HeLa cells. Coexpression of Rab11wt-GFP (B) or Rab11QL-GFP (C) greatly reduced hEcad surface delivery, so that no surface labeling was detectable. In these same cells, hEcad was concentrated in intracellular structures, many of which also contained either Rab11wt-GFP or Rab11QL-GFP. Similar results were observed in preconfluent MDCK cells expressing hEcad and Rab11QL-GFP, with hEcad surface delivery blocked, and strong intracellular accumulation in enlarged Rab11QL-GFP–positive compartments (D). In contrast to hEcad, ΔS1-Ecad was efficiently transported to the cell surface of HeLa cells in the presence of Rab11QL-GFP (F) and was colocalized in intracellular structures with Rab11QL-GFP. Scale bars, 10 μm.
Figure 7.
Rab11 mutants mistarget hEcad and transform polarized MDCK cells. Confluent monolayers of MDCK cells were fixed and labeled, and confocal imaging was performed to generate XZ and XY sections. Single expression of hEcad (A) or ΔS1-Ecad (B) shows their basolateral or apical distribution, respectively (red labeling), relative to nuclear staining (DAPI, blue). In cells coexpressing Rab11SN-GFP (green), hEcad (red) is dramatically shifted to the apical surface (C). Coexpression of Rab11SN-GFP (green) does not alter the apical delivery of ΔS1-Ecad (red; D). The expression of Rab11SN-GFP notably transforms the cells initially causing enhanced spreading and invasive protrusion at the basal cell surface, as seen in basal XY sections (C and D). This phenotypic change was even more striking after expression of Rab11QL-GFP as seen in a 3D reconstruction of cells cotransfected with Rab11QL-GFP (green) and hEcad (red; E). 3D rendered animations of confocal sections pertaining to each panel are available in the supplementary material (Supplementary Movies 5–9) Scale bars, 10 μm.
Similar articles
- Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis.
Desclozeaux M, Venturato J, Wylie FG, Kay JG, Joseph SR, Le HT, Stow JL. Desclozeaux M, et al. Am J Physiol Cell Physiol. 2008 Aug;295(2):C545-56. doi: 10.1152/ajpcell.00097.2008. Epub 2008 Jun 25. Am J Physiol Cell Physiol. 2008. PMID: 18579802 - Anterograde trafficking of KCa3.1 in polarized epithelia is Rab1- and Rab8-dependent and recycling endosome-independent.
Bertuccio CA, Lee SL, Wu G, Butterworth MB, Hamilton KL, Devor DC. Bertuccio CA, et al. PLoS One. 2014 Mar 14;9(3):e92013. doi: 10.1371/journal.pone.0092013. eCollection 2014. PLoS One. 2014. PMID: 24632741 Free PMC article. - Rab6 Is Required for Multiple Apical Transport Pathways but Not the Basolateral Transport Pathway in Drosophila Photoreceptors.
Iwanami N, Nakamura Y, Satoh T, Liu Z, Satoh AK. Iwanami N, et al. PLoS Genet. 2016 Feb 18;12(2):e1005828. doi: 10.1371/journal.pgen.1005828. eCollection 2016 Feb. PLoS Genet. 2016. PMID: 26890939 Free PMC article. - Orchestration of cell surface proteins by Rab11.
Welz T, Wellbourne-Wood J, Kerkhoff E. Welz T, et al. Trends Cell Biol. 2014 Jul;24(7):407-15. doi: 10.1016/j.tcb.2014.02.004. Epub 2014 Mar 24. Trends Cell Biol. 2014. PMID: 24675420 Review. - Polarized endocytic transport: the roles of Rab11 and Rab11-FIPs in regulating cell polarity.
Jing J, Prekeris R. Jing J, et al. Histol Histopathol. 2009 Sep;24(9):1171-80. doi: 10.14670/HH-24.1171. Histol Histopathol. 2009. PMID: 19609864 Free PMC article. Review.
Cited by
- BEACH domain proteins function as cargo-sorting adaptors in secretory and endocytic pathways.
Pankiv S, Dahl AK, Aas A, Andersen RL, Brech A, Holland P, Singh S, Bindesbøll C, Simonsen A. Pankiv S, et al. J Cell Biol. 2024 Dec 2;223(12):e202408173. doi: 10.1083/jcb.202408173. Epub 2024 Nov 8. J Cell Biol. 2024. PMID: 39514288 Free PMC article. - ARF1 compartments direct cargo flow via maturation into recycling endosomes.
Stockhammer A, Adarska P, Natalia V, Heuhsen A, Klemt A, Bregu G, Harel S, Rodilla-Ramirez C, Spalt C, Özsoy E, Leupold P, Grindel A, Fox E, Mejedo JO, Zehtabian A, Ewers H, Puchkov D, Haucke V, Bottanelli F. Stockhammer A, et al. Nat Cell Biol. 2024 Nov;26(11):1845-1859. doi: 10.1038/s41556-024-01518-4. Epub 2024 Oct 4. Nat Cell Biol. 2024. PMID: 39367144 Free PMC article. - Transport mechanisms between the endocytic, recycling, and biosynthetic pathways via endosomes and the _trans_-Golgi network.
Toshima JY, Toshima J. Toshima JY, et al. Front Cell Dev Biol. 2024 Sep 3;12:1464337. doi: 10.3389/fcell.2024.1464337. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39291266 Free PMC article. Review. - A novel noncanonical function for IRF6 in the recycling of E-cadherin.
Antiguas A, Dunnwald M. Antiguas A, et al. Mol Biol Cell. 2024 Jul 1;35(7):ar102. doi: 10.1091/mbc.E23-11-0430. Epub 2024 May 29. Mol Biol Cell. 2024. PMID: 38809584 Free PMC article. - A fly's eye view of quiescent neural stem cells.
Gujar MR, Wang H. Gujar MR, et al. Oxf Open Neurosci. 2022 May 4;1:kvac001. doi: 10.1093/oons/kvac001. eCollection 2022. Oxf Open Neurosci. 2022. PMID: 38596705 Free PMC article. Review.
References
- Band, A. M., Ali, H., Vartiainen, M. K., Welti, S., Lappalainen, P., Olkkonen, V. M., and Kuismanen, E. (2002). Endogenous plasma membrane t-SNARE syntaxin 4 is present in rab11 positive endosomal membranes and associates with cortical actin cytoskeleton. FEBS Lett. 531, 513–519. - PubMed
- Bonifacino, J. S., and Lippincott-Schwartz, J. (2003). Coat proteins: shaping membrane transport. Nat. Rev. Mol. Cell. Biol. 4, 409–414. - PubMed
- Bryant, D. M., and Stow, J. L. (2004). The ins and outs of E-cadherin trafficking. Trends Cell Biol. 14, 427–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases