Defective neuromuscular synapses in mice lacking amyloid precursor protein (APP) and APP-Like protein 2 - PubMed (original) (raw)
Defective neuromuscular synapses in mice lacking amyloid precursor protein (APP) and APP-Like protein 2
Pei Wang et al. J Neurosci. 2005.
Abstract
Biochemical and genetic studies place the amyloid precursor protein (APP) at the center stage of Alzheimer's disease (AD) pathogenesis. Although mutations in the APP gene lead to dominant inheritance of familial AD, the normal function of APP remains elusive. Here, we report that the APP family of proteins plays an essential role in the development of neuromuscular synapses. Mice deficient in APP and its homolog APP-like protein 2 (APLP2) exhibit aberrant apposition of presynaptic marker proteins with postsynaptic acetylcholine receptors and excessive nerve terminal sprouting. The number of synaptic vesicles at presynaptic terminals is dramatically reduced. These structural abnormalities are accompanied by defective neurotransmitter release and a high incidence of synaptic failure. Our results identify APP/APLP2 as key regulators of structure and function of developing neuromuscular synapses.
Figures
Figure 1.
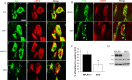
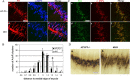
Characterization and quantification of P0 sternomastoid muscles. A, Double labeling with the anti-Syn antibody and α-BTX of WT, APP-/-, APLP2-/-, and APP/APLP2 double knock-out (dKO) animals. Scale bar, 5 μm. B, SV2 and α-BTX double staining of littermate APLP2-/- and dKO NMJs. The arrowheads in d and f mark the SV2 staining beyond the end plate. C, Quantification of the percentage of AChR-positive end plates covered by SV2 immunoreactivity (average ± SD of 20 end plates per genotype). *p < 0.01; t test. D, Western blot analysis of P0 spinal cord proteins using an anti-APP C-terminal antibody (APP) or anti-SV2 antibody (SV2). Anti-tubulin (Tubulin) Western blot was used as the loading control. The images were captured by a confocal microscope and displayed either as individual staining or merged images.
Figure 2.
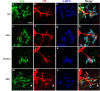
Triple labeling of P0 WT, APP-/-, APLP2-/-, and APP/APLP2 dKO sternomastoid muscles with anti-Syn antibody, anti-NF antibody, and α-BTX. The arrowheads in m, n, and p denote a representative Syn-positive nerve sprouted beyond the end plate. The images were captured by a confocal microscope and displayed either as individual staining or merged images. Scale bar, 20 μm.
Figure 3.
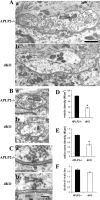
EM analysis of presynaptic terminal structures of P0 sternomastoid muscles. A, B, Representative EM images showing reduced synaptic vesicles in APP/APLP2 dKO terminals compared with littermate APLP2-/- control terminals of similar sizes. C, Representative electron-dense active zones, which could be identified in both genotypes. D, Quantification of synaptic vesicle densities (number of vesicles per square micrometer of profile area). *p < 0.001; Student's t test. E, Quantification of the number of active zones per micrometer of profile length. *p < 0.001; Student's t test. F, Number of docked vesicles per active zone. All calculations were done on a per section basis. Each column represents mean ± SE of 40 profiles from two animals. Scale bars: A, B, 1 μm; C, 200 μm.
Figure 4.
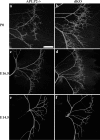
Whole-mount NF staining of P0 (a, b), E16.5 (c, d), and E14.5 (e, f) diaphragm of littermate APLP2-/- control and APP/APLP2 dKO is shown. Nerve terminal sprouting is apparent in E16.5 and P0 but not in E14.5 dKO samples. Scale bar, 400 μm.
Figure 5.
Examination of synaptic distribution of the diaphragm muscle. A, Double labeling with the anti-NF antibody (a, d) and α-BTX (b, e), revealing excessive terminal sprouting and widened AChR-positive end plate band in APP/APLP2 dKO animals. B, Quantification of AChR cluster distribution from the medial edge of the diaphragm (mean ± SD of 3 animals per genotype). *p < 0.05; t test. C, Syn and α-BTX double staining, which showed broadened presynaptic distribution. D, Acetylcholine esterase histochemistry, documenting diffused synaptic patterning. The right columns in A and C are the merged images of the first two columns. Scale bars: A, 50 μm; C, D, 100 μm.
Figure 6.
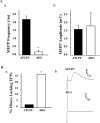
Electrophysiological recordings from P0 sternomastoid muscle fibers of APP/APLP2 double null (dKO) mice and control littermates (APLP2-/-). A, MEPP recordings. Aa, Reduced MEPP frequency (mean ± SD) in dKO (n = 8) compared with control (n = 7); *p < 0.001. Over one-half of the fibers in this analysis did not have detectable MEPPs. Ab, Similar MEPP amplitude in APLP2-/- control and APP/APLP2 null (dKO) mutant (mean ± SD); p = 0.23. B, EPP recordings. Ba, Percentage of fibers in which an evoked response (EPP or action potential) could not be induced by supramaximal nerve stimulation. Control (APLP2-/-) fibers, 1 of 41; double null (dKO) fibers, 8 of 31. *p < 0.005. Bb, Representative EPP evoked in a sternomastoid fiber by stimulation of the muscle nerve, with μ-conotoxin present in the bath. Top trace, EPP from an APLP2-/- control; bottom trace, EPP from a littermate mutant (dKO), at 10 times the threshold required to evoke responses in adjacent muscle fibers.
Similar articles
- Differential role of APP and APLPs for neuromuscular synaptic morphology and function.
Klevanski M, Saar M, Baumkötter F, Weyer SW, Kins S, Müller UC. Klevanski M, et al. Mol Cell Neurosci. 2014 Jul;61:201-10. doi: 10.1016/j.mcn.2014.06.004. Epub 2014 Jul 4. Mol Cell Neurosci. 2014. PMID: 24998676 - Reduced synaptic vesicle density and active zone size in mice lacking amyloid precursor protein (APP) and APP-like protein 2.
Yang G, Gong YD, Gong K, Jiang WL, Kwon E, Wang P, Zheng H, Zhang XF, Gan WB, Zhao NM. Yang G, et al. Neurosci Lett. 2005 Aug 12-19;384(1-2):66-71. doi: 10.1016/j.neulet.2005.04.040. Neurosci Lett. 2005. PMID: 15919150 - Amyloid Precursor-Like Protein 2 deletion-induced retinal synaptopathy related to congenital stationary night blindness: structural, functional and molecular characteristics.
Dinet V, Ciccotosto GD, Delaunay K, Borras C, Ranchon-Cole I, Kostic C, Savoldelli M, El Sanharawi M, Jonet L, Pirou C, An N, Abitbol M, Arsenijevic Y, Behar-Cohen F, Cappai R, Mascarelli F. Dinet V, et al. Mol Brain. 2016 Jun 8;9(1):64. doi: 10.1186/s13041-016-0245-z. Mol Brain. 2016. PMID: 27267879 Free PMC article. - The role of APP and APLP for synaptic transmission, plasticity, and network function: lessons from genetic mouse models.
Korte M, Herrmann U, Zhang X, Draguhn A. Korte M, et al. Exp Brain Res. 2012 Apr;217(3-4):435-40. doi: 10.1007/s00221-011-2894-6. Epub 2011 Oct 18. Exp Brain Res. 2012. PMID: 22006270 Review. - Roles of the amyloid precursor protein family in the peripheral nervous system.
Caldwell JH, Klevanski M, Saar M, Müller UC. Caldwell JH, et al. Mech Dev. 2013 Jun-Aug;130(6-8):433-46. doi: 10.1016/j.mod.2012.11.001. Epub 2012 Nov 29. Mech Dev. 2013. PMID: 23201910 Review.
Cited by
- Enhanced β-secretase processing alters APP axonal transport and leads to axonal defects.
Rodrigues EM, Weissmiller AM, Goldstein LS. Rodrigues EM, et al. Hum Mol Genet. 2012 Nov 1;21(21):4587-601. doi: 10.1093/hmg/dds297. Epub 2012 Jul 27. Hum Mol Genet. 2012. PMID: 22843498 Free PMC article. - Molecular mechanism of active zone organization at vertebrate neuromuscular junctions.
Nishimune H. Nishimune H. Mol Neurobiol. 2012 Feb;45(1):1-16. doi: 10.1007/s12035-011-8216-y. Epub 2011 Dec 2. Mol Neurobiol. 2012. PMID: 22135013 Free PMC article. Review. - Intracellular trafficking and synaptic function of APL-1 in Caenorhabditis elegans.
Wiese M, Antebi A, Zheng H. Wiese M, et al. PLoS One. 2010 Sep 20;5(9):e12790. doi: 10.1371/journal.pone.0012790. PLoS One. 2010. PMID: 20862215 Free PMC article. - APP anterograde transport requires Rab3A GTPase activity for assembly of the transport vesicle.
Szodorai A, Kuan YH, Hunzelmann S, Engel U, Sakane A, Sasaki T, Takai Y, Kirsch J, Müller U, Beyreuther K, Brady S, Morfini G, Kins S. Szodorai A, et al. J Neurosci. 2009 Nov 18;29(46):14534-44. doi: 10.1523/JNEUROSCI.1546-09.2009. J Neurosci. 2009. PMID: 19923287 Free PMC article. - Essential roles for the FE65 amyloid precursor protein-interacting proteins in brain development.
Guénette S, Chang Y, Hiesberger T, Richardson JA, Eckman CB, Eckman EA, Hammer RE, Herz J. Guénette S, et al. EMBO J. 2006 Jan 25;25(2):420-31. doi: 10.1038/sj.emboj.7600926. Epub 2006 Jan 12. EMBO J. 2006. PMID: 16407979 Free PMC article.
References
- Akaaboune M, Allinquant B, Farza H, Roy K, Magoul R, Fiszman M, Festoff BW, Hantai D (2000) Developmental regulation of amyloid precursor protein at the neuromuscular junction in mouse skeletal muscle. Mol Cell Neurosci 15: 355-367. - PubMed
- Cao X, Sudhof TC (2001) A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293:115-120. - PubMed
- Dawson GR, Seabrook GR, Zheng H, Smith DW, Graham S, O'Dowd G, Bowery BJ, Boyce S, Trumbauer ME, Chen HY, Van der Ploeg LH, Sirinathsinghji DJ (1999) Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein [In Process Citation]. Neuroscience 90: 1-13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS041846/NS/NINDS NIH HHS/United States
- R01 NS041846/NS/NINDS NIH HHS/United States
- R01 AG021141/AG/NIA NIH HHS/United States
- NS40039/NS/NINDS NIH HHS/United States
- R01 NS040039/NS/NINDS NIH HHS/United States
- AG20670/AG/NIA NIH HHS/United States
- R01 AG020670/AG/NIA NIH HHS/United States
- AG21141/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases