Rab27a: a new face in beta cell metabolism-secretion coupling - PubMed (original) (raw)
Review
Rab27a: a new face in beta cell metabolism-secretion coupling
Toru Aizawa et al. J Clin Invest. 2005 Feb.
Abstract
In pancreatic beta cells, not only insulin exocytosis per se, but translocation of beta granules toward the plasma membrane--an event upstream of exocytosis--are under the control of glucose. However, the molecular basis of this translocation has been poorly understood. Rab27a-mediated translocation of glucose-induced beta granules is reported in this issue of the JCI. Rab27a or its effector molecule may constitute a novel pharmacological target because potentiation of the Rab27a pathway is expected to restore beta cell glucose competency in patients with diabetes mellitus.
Figures
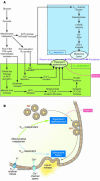
Figure 1
Metabolism-secretion coupling within the pancreatic β cell. (A and B) Elevation of ATP and ATP/ADP is the starting point for KATP channel–dependent signaling, which has been well elucidated, as shown here (6). Owing to [Ca2+]i elevation, fusion of β granules in the RRP and the plasma membrane takes place, and this results in the first phase of glucose-induced insulin secretion. The molecular basis for KATP channel–independent signaling (B) is not fully characterized. The end point of this signaling is expansion and/or replenishment of the RRP, after which a second phase occurs in which insulin release gradually increases. Upon glucose stimulation, KATP channel–dependent and –independent events take place. When an experimental protocol to detect time-dependent potentiation is employed (16, 17), expansion of the RRP can be quantified. Membrane fusion of the β granules in the RRP is required and sufficient for first-phase insulin release, and, in addition, expansion and/or replenishment of the RRP is required for second phase release (see Figure 2). As shown in A, as a result of anaplerosis (2), increased citrate flux (2) and/or activation of acetyl-CoA carboxylase (ACC) (3) occurs, which results in expansion and/or replenishment of the RRP via the series of events indicated (10–12). Cytosolic flux of glutamate, by virtue of its uptake by β granules, sensitizes the granules for fusion (15). Activation of phospholipase C (PLC), PKC, or an increase in inositol trisphosphate (IP3) (4) and liberation of stored Ca2+ (13) also yield expansion and/or replenishment. ATP itself (14) and elevation of ATP/ADP (9) are also involved in this process. In this issue of the JCI, Kasai et al. (1) report that Rab27a plays a critical role in replenishment of the RRP. CPT1, carnitine palmitoyl transferase 1; L-VDCC, L-type voltage-dependent Ca2+ channel.
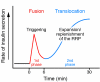
Figure 2
A schematic presentation of glucose-induced biphasic insulin secretion by islet β cells. Stimulation by a high concentration of glucose elicits a rapid increase in insulin secretion within 1–2 minutes (first phase). Within 10 minutes, in the presence of the same stimulatory concentration of glucose, the secretion rate is decreased, and it gradually begins to increase again thereafter (second phase). Second-phase insulin release is more prominent in rat and human than in mouse β cells. First-phase insulin release is generated by fusion of β granules already in the RRP and the plasma membrane, which is called triggering. For the second phase, granules translocate from the RP to the RRP, which yields expansion and/or replenishment of the RRP. The first and second phases of insulin secretion are due to KATP channel–dependent and –independent glucose signaling, respectively, as outlined in Figure 1.
Comment on
- Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation.
Kasai K, Ohara-Imaizumi M, Takahashi N, Mizutani S, Zhao S, Kikuta T, Kasai H, Nagamatsu S, Gomi H, Izumi T. Kasai K, et al. J Clin Invest. 2005 Feb;115(2):388-96. doi: 10.1172/JCI22955. J Clin Invest. 2005. PMID: 15690086 Free PMC article.
Similar articles
- Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation.
Kasai K, Ohara-Imaizumi M, Takahashi N, Mizutani S, Zhao S, Kikuta T, Kasai H, Nagamatsu S, Gomi H, Izumi T. Kasai K, et al. J Clin Invest. 2005 Feb;115(2):388-96. doi: 10.1172/JCI22955. J Clin Invest. 2005. PMID: 15690086 Free PMC article. - The Rab27a effector exophilin7 promotes fusion of secretory granules that have not been docked to the plasma membrane.
Wang H, Ishizaki R, Xu J, Kasai K, Kobayashi E, Gomi H, Izumi T. Wang H, et al. Mol Biol Cell. 2013 Feb;24(3):319-30. doi: 10.1091/mbc.E12-04-0265. Epub 2012 Dec 5. Mol Biol Cell. 2013. PMID: 23223571 Free PMC article. - The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules.
Yi Z, Yokota H, Torii S, Aoki T, Hosaka M, Zhao S, Takata K, Takeuchi T, Izumi T. Yi Z, et al. Mol Cell Biol. 2002 Mar;22(6):1858-67. doi: 10.1128/MCB.22.6.1858-1867.2002. Mol Cell Biol. 2002. PMID: 11865063 Free PMC article. - Rab27a in pancreatic beta-cells, a busy protein in membrane trafficking.
Kimura T, Niki I. Kimura T, et al. Prog Biophys Mol Biol. 2011 Nov;107(2):219-23. doi: 10.1016/j.pbiomolbio.2011.06.016. Epub 2011 Jul 7. Prog Biophys Mol Biol. 2011. PMID: 21762718 Review. - Rab27a, actin and beta-cell endocytosis.
Kimura T, Niki I. Kimura T, et al. Endocr J. 2011;58(1):1-6. doi: 10.1507/endocrj.k10e-391. Epub 2010 Dec 23. Endocr J. 2011. PMID: 21187662 Review.
Cited by
- Dysglycemia With Impaired Insulin Secretion After Resection of a High-Molecular-Weight IGF-II-Producing Tumor.
Shimada Y, Yamashita K, Fukuda I, Aizawa T. Shimada Y, et al. JCEM Case Rep. 2022 Nov 29;1(1):luac013. doi: 10.1210/jcemcr/luac013. eCollection 2023 Jan. JCEM Case Rep. 2022. PMID: 37908273 Free PMC article. - Sirolimus induces depletion of intracellular calcium stores and mitochondrial dysfunction in pancreatic beta cells.
Lombardi A, Gambardella J, Du XL, Sorriento D, Mauro M, Iaccarino G, Trimarco B, Santulli G. Lombardi A, et al. Sci Rep. 2017 Nov 20;7(1):15823. doi: 10.1038/s41598-017-15283-y. Sci Rep. 2017. PMID: 29158477 Free PMC article. - Hypertonicity during a rapid rise in D-glucose mediates first-phase insulin secretion.
Kamat V, Sweet IR. Kamat V, et al. Front Endocrinol (Lausanne). 2024 Jun 26;15:1395028. doi: 10.3389/fendo.2024.1395028. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38989001 Free PMC article. - In vitro expression of NGN3 identifies RAB3B as the predominant Ras-associated GTP-binding protein 3 family member in human islets.
Piper Hanley K, Hearn T, Berry A, Carvell MJ, Patch AM, Williams LJ, Sugden SA, Wilson DI, Ellard S, Hanley NA. Piper Hanley K, et al. J Endocrinol. 2010 Nov;207(2):151-61. doi: 10.1677/JOE-10-0120. Epub 2010 Aug 31. J Endocrinol. 2010. PMID: 20807725 Free PMC article. - Role of G-proteins in islet function in health and diabetes.
Kowluru A. Kowluru A. Diabetes Obes Metab. 2017 Sep;19 Suppl 1(Suppl 1):63-75. doi: 10.1111/dom.13011. Diabetes Obes Metab. 2017. PMID: 28880478 Free PMC article. Review.
References
- Farfari S, Schulz V, Corkey B, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes. 2000;49:718–726. - PubMed
- Zawalich WS, Zawalich KC. Regulation of insulin secretion by phospholipase C. Am. J. Physiol. 1996;271:E409–E416. - PubMed
- Nicholls LI, Ainscow EK, Rutter GA. Glucose-stimulated insulin secretion does not require activation of pyruvate dehydrogenase: impact of adenovirus-mediated overexpression of PDH kinase and PDH phosphate phosphatase in pancreatic islets. Biochem. Biophys. Res. Commun. 2002;291:1081–1088. - PubMed