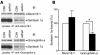Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation - PubMed (original) (raw)
Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation
Kazuo Kasai et al. J Clin Invest. 2005 Feb.
Abstract
The monomeric small GTPase Rab27a is specifically localized on both secretory granules and lysosome-related organelles. Although natural mutations of the Rab27a gene in human Griscelli syndrome and in ashen mice cause partial albinism and immunodeficiency reflecting the dysfunction of lysosome-related organelles, phenotypes resulting from the defective exocytosis of secretory granules have not been reported. To explore the roles of Rab27a in secretory granules, we analyzed insulin secretion profiles in ashen mice. Ashen mice showed glucose intolerance after a glucose load without signs of insulin resistance in peripheral tissues or insulin deficiency in the pancreas. Insulin secretion from isolated islets was decreased specifically in response to high glucose concentrations but not other nonphysiological secretagogues such as high K+ concentrations, forskolin, or phorbol ester. Neither the intracellular Ca2+ concentration nor the dynamics of fusion pore opening after glucose stimulation were altered. There were, however, marked reductions in the exocytosis from insulin granules predocked on the plasma membrane and in the replenishment of docked granules during glucose stimulation. These results provide the first genetic evidence to our knowledge for the role of Rab27a in the exocytosis of secretory granules and suggest that the Rab27a/effector system mediates glucose-specific signals for the exocytosis of insulin granules in pancreatic beta cells.
Figures
Figure 1
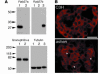
Expression of Rab27a, Rab27b, and granuphilin in pancreatic islets. (A) An equal amount of protein (20 μg) from the pancreatic islets of 17-week-old male C3H/He (lane 1) and ashen mice (lane 2) was separated by electrophoresis for immunoblotting with anti-Rab27a, anti-Rab27b, or anti-granuphilin (αGrp-aC) antibodies. The expression levels of α-tubulin were also examined for normalization. For the immunoblotting with anti-Rab27b and anti–α-tubulin antibodies, 20 μg of protein from the pituitary of C3H/He mice were loaded on lane 3 for the reference. Numbers to the left of each panel are molecular masses in kDa. (B) The pancreas organs of 17-week-old male C3H/He (upper) or ashen mice (lower) were immunostained with anti-granuphilin antibodies (αGrp-N). Granuphilin is distinguishably concentrated along the plasma membrane in ashen β cells (arrowheads) compared with control β cells, although the expression levels are similar. Scale bar: 20 μm.
Figure 2
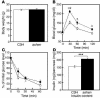
In vivo phenotypes of ashen and C3H/He mice. All the phenotypes were derived from male C3H/He (white bars or open circles) or ashen (black bars or filled circles) mice. Body weight (A), blood glucose concentrations during an intraperitoneal glucose tolerance test (B), and total insulin content in the pancreas (D) were measured at 15 weeks of age. An intraperitoneal insulin tolerance test (C) was performed at 16 weeks of age, and its results are expressed as a percentage of the initial blood glucose concentration. Values are mean ± SE (A and B, n = 8 for C3H/He, n = 7 for ashen; C, n = 5 for C3H/He and ashen; D, n = 8 for C3H/He and ashen). The statistical significance of differences between means was assessed by Student’s t test. *P < 0.05, **P < 0.005, ***P < 0.0005 vs. C3H/He mice.
Figure 3
Insulin secretion profiles of perifused islets. Insulin secretion was examined in islets isolated from age-matched (15–17 weeks of age) male C3H/He (open symbols) or ashen mice (filled symbols). The islets were stabilized by perifusion of standard low-glucose (2.8 mM) Krebs-Ringer buffer for 10 minutes, after which an appropriate secretagogue was applied. (A and B) Islets were stimulated by 16.7 mM glucose for 30 minutes (A; n = 9 for each strain) or with 60 mM KCl for 15 minutes (B; n = 6). (C and D) Islets were stimulated by 16.7 mM glucose for 30 minutes in the continuous presence of either forskolin (C, 10 mM; n = 8) or PMA (D, 0.5 mM; n = 8). (E and F) Islets were stimulated 4 times by either 16.7 mM glucose for 20 minutes (E; n = 3) or by 60 mM KCl for 10 minutes (F; n = 3), with 10-minute intervals of the standard buffer. (G) Islets were stimulated by 60 mM (squares), 30 mM (circles), or 20 mM KCl (triangles) for 15 minutes, followed by the standard buffer for 15 minutes (n = 3 for each condition and strain). (H) Islets were perifused with buffer containing 250 μM diazoxide and 16.7 mM glucose for 10 minutes. They were further perifused with the buffer containing 30 mM KCl for 30 minutes, in the continuous presence of diazoxide and glucose (n = 3 for each strain). Values are mean ± SE.
Figure 4
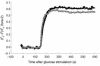
The rise in cytosolic Ca2+ concentration in response to glucose stimulation. The cytosolic Ca2+ concentration was measured using Fura-2 acetoxymethyl ester by two-photon excitation imaging in pancreatic β cells of either C3H/He (gray) or ashen (black) mice and was represented by (F_0–_F)/_F_0, where _F_0 and F stand for resting and fluorescence and fluorescence after 20 mM glucose stimulation, respectively. Mean values are shown (n = 4).
Figure 5
TIRFM analysis of the exocytosis of insulin granules. (A) Pancreatic β cells from either C3H/He (left) or ashen (right) mice were fixed, immunostained with anti-insulin antibodies, and observed by TIRFM. The surrounding lines represent the outline of cells that are attached to the cover glass. Scale bar: 5 μm. (B) Pancreatic β cells of either C3H/He (upper) or ashen (lower) mice were infected with adenoviruses encoding insulin-GFP. Evanescent images in live cells were acquired every 300 milliseconds after glucose stimulation. The fusion events per 200 μm2 were manually counted. The histograms show the number of fusion events at 60-second intervals after high-glucose (16.7 mM) stimulation. The black bars show the fusion from previously docked granules, whereas the white bars represent that from newly recruited granules. Values are mean ± SE (n = 24 for ashen and n = 21 for C3H/He mice). The statistical significance of differences between means were assessed by repeated-measures ANOVA. (C) The number of morphologically docked granules was counted on the TIRFM images of pancreatic β cells that had been infected with adenoviruses encoding insulin-GFP. The number after a glucose stimulation (16.7 mM, 15 minutes) was normalized to that prior to the stimulation in the identical area (200 μm2) of C3H/He (open circles) or ashen (filled circles) β cells. Values are mean ± SE (n = 4). The statistical significance of differences between means was assessed by Student’s t test. *P < 0.0005 vs. C3H/He mice.
Figure 6
Ultrastructure of the pancreatic β cells. (A) Electron micrographs of β cells were taken in nonstimulated (upper) or glucose-stimulated (lower) islets of C3H/He (left) and ashen (right) mice. Scale bar: 1 μm. (B) Relative density of granules below the plasma membrane in nonstimulated (white bars) or glucose-stimulated (black bars) β cells of C3H/He (left) and ashen (right) mice is shown as a function of the distance from granule center to the plasma membrane (nm). Data are represented as a percentage of the granule density in each concentric shell below the plasma membrane relative to the average density in cytoplasm (100% = number of total granules per the area of cytoplasm, that is, the cell area minus the nuclear area). Values are mean ± SE (n = 10). The statistical significance of differences between means was assessed by Student’s t test. *P < 0.05.
Figure 7
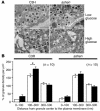
Protein interactions of syntaxin 1a in pancreatic islets. (A) Protein interactions of syntaxin 1a with either Munc18-1 (upper) or granuphilin-a (lower) were analyzed in extracts of C3H/He or ashen islets. The amount of Munc18-1, granuphilin-a, and syntaxin 1a in each lysate (5 μg, left) and 30–40% of the immunoprecipitates of Munc18-1 or granuphilin-a (right) were examined by immunoblotting with the antibodies indicated. (B) Results of coimmunoprecipitation experiments independently performed as described in A were gathered for statistics. Relative intensities of syntaxin 1a signals in ashen mice (black bars) to those in C3H/He mice (white bars) were calculated and expressed as mean ± SE from 7 (vs. Munc18-1) and 6 (vs. granuphilin-a) immunoblot preparations. When analyzed by a Wilcoxon signed-ranks test, ashen islets showed significantly reduced interaction of syntaxin 1a with granuphilin-a (*P = 0.027), but not with Munc18-1.
Comment in
- Rab27a: a new face in beta cell metabolism-secretion coupling.
Aizawa T, Komatsu M. Aizawa T, et al. J Clin Invest. 2005 Feb;115(2):227-30. doi: 10.1172/JCI24269. J Clin Invest. 2005. PMID: 15690078 Free PMC article. Review.
Similar articles
- The Rab27a effector exophilin7 promotes fusion of secretory granules that have not been docked to the plasma membrane.
Wang H, Ishizaki R, Xu J, Kasai K, Kobayashi E, Gomi H, Izumi T. Wang H, et al. Mol Biol Cell. 2013 Feb;24(3):319-30. doi: 10.1091/mbc.E12-04-0265. Epub 2012 Dec 5. Mol Biol Cell. 2013. PMID: 23223571 Free PMC article. - The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules.
Yi Z, Yokota H, Torii S, Aoki T, Hosaka M, Zhao S, Takata K, Takeuchi T, Izumi T. Yi Z, et al. Mol Cell Biol. 2002 Mar;22(6):1858-67. doi: 10.1128/MCB.22.6.1858-1867.2002. Mol Cell Biol. 2002. PMID: 11865063 Free PMC article. - The Rab-binding protein Noc2 is associated with insulin-containing secretory granules and is essential for pancreatic beta-cell exocytosis.
Cheviet S, Coppola T, Haynes LP, Burgoyne RD, Regazzi R. Cheviet S, et al. Mol Endocrinol. 2004 Jan;18(1):117-26. doi: 10.1210/me.2003-0300. Epub 2003 Oct 30. Mol Endocrinol. 2004. PMID: 14593078 - Rab27a: a new face in beta cell metabolism-secretion coupling.
Aizawa T, Komatsu M. Aizawa T, et al. J Clin Invest. 2005 Feb;115(2):227-30. doi: 10.1172/JCI24269. J Clin Invest. 2005. PMID: 15690078 Free PMC article. Review. - Heterogeneous modes of insulin granule exocytosis: molecular determinants.
Izumi T. Izumi T. Front Biosci (Landmark Ed). 2011 Jan 1;16(1):360-7. doi: 10.2741/3692. Front Biosci (Landmark Ed). 2011. PMID: 21196175 Review.
Cited by
- Locked and Loaded: Mechanisms Regulating Natural Killer Cell Lytic Granule Biogenesis and Release.
Ham H, Medlyn M, Billadeau DD. Ham H, et al. Front Immunol. 2022 Apr 26;13:871106. doi: 10.3389/fimmu.2022.871106. eCollection 2022. Front Immunol. 2022. PMID: 35558071 Free PMC article. Review. - Molecular mechanism of docking of dense-core vesicles to the plasma membrane in neuroendocrine cells.
Tsuboi T. Tsuboi T. Med Mol Morphol. 2008 Jun;41(2):68-75. doi: 10.1007/s00795-008-0400-4. Epub 2008 Jul 1. Med Mol Morphol. 2008. PMID: 18592160 Review. - Rab GTPases implicated in inherited and acquired disorders.
Mitra S, Cheng KW, Mills GB. Mitra S, et al. Semin Cell Dev Biol. 2011 Feb;22(1):57-68. doi: 10.1016/j.semcdb.2010.12.005. Epub 2010 Dec 13. Semin Cell Dev Biol. 2011. PMID: 21147240 Free PMC article. Review. - Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins.
Wang Z, Thurmond DC. Wang Z, et al. J Cell Sci. 2009 Apr 1;122(Pt 7):893-903. doi: 10.1242/jcs.034355. J Cell Sci. 2009. PMID: 19295123 Free PMC article. Review. - Regulation of insulin granule turnover in pancreatic beta-cells by cleaved ICA512.
Trajkovski M, Mziaut H, Schubert S, Kalaidzidis Y, Altkrüger A, Solimena M. Trajkovski M, et al. J Biol Chem. 2008 Nov 28;283(48):33719-29. doi: 10.1074/jbc.M804928200. Epub 2008 Sep 29. J Biol Chem. 2008. PMID: 18824546 Free PMC article.
References
- Izumi T, Gomi H, Kasai K, Mizutani S, Torii S. The roles of Rab27 and its effectors in the regulated secretory pathways. Cell Struct. Funct. 2003;28:465–474. - PubMed
- Söllner TH. Regulated exocytosis and SNARE function. Mol. Membr. Biol. 2003;20:209–220. - PubMed
- Blott EJ, Griffiths GM. Secretory lysosomes. Nat. Rev. Mol. Cell Biol. 2002;3:122–131. - PubMed
- Nagashima K, et al. Melanophilin directly links Rab27a and myosin Va through its distinct coiled-coil regions. FEBS Lett. 2002;517:233–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous