The splicing regulatory element, UGCAUG, is phylogenetically and spatially conserved in introns that flank tissue-specific alternative exons - PubMed (original) (raw)
The splicing regulatory element, UGCAUG, is phylogenetically and spatially conserved in introns that flank tissue-specific alternative exons
Simon Minovitsky et al. Nucleic Acids Res. 2005.
Abstract
Previous studies have identified UGCAUG as an intron splicing enhancer that is frequently located adjacent to tissue-specific alternative exons in the human genome. Here, we show that UGCAUG is phylogenetically and spatially conserved in introns that flank brain-enriched alternative exons from fish to man. Analysis of sequence from the mouse, rat, dog, chicken and pufferfish genomes revealed a strongly statistically significant association of UGCAUG with the proximal intron region downstream of brain-enriched alternative exons. The number, position and sequence context of intronic UGCAUG elements were highly conserved among mammals and in chicken, but more divergent in fish. Control datasets, including constitutive exons and non-tissue-specific alternative exons, exhibited a much lower incidence of closely linked UGCAUG elements. We propose that the high sequence specificity of the UGCAUG element, and its unique association with tissue-specific alternative exons, mark it as a critical component of splicing switch mechanism(s) designed to activate a limited repertoire of splicing events in cell type-specific patterns. We further speculate that highly conserved UGCAUG-binding protein(s) related to the recently described Fox-1 splicing factor play a critical role in mediating this specificity.
Figures
Figure 1
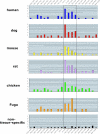
Enrichment of candidate UGCAUG alternative splicing enhancers in the proximal downstream intron region is conserved among vertebrate species. Histograms show the frequency of UGCAUG elements at 100 nt intervals in the introns upstream and downstream of the brain-enriched exons. Top six panels represent the analysis of intron sequences for the vertebrate species indicated at the left. Note the highest abundance of UGCAUG elements is consistently within the downstream ∼400 nt (D400) region. The bottom panel shows the much lower incidence of UGCAUG elements that occurs near a group of non-tissue-specific alternative exons. Vertical axis, frequency; horizontal axis, nucleotide range relative to the alternative exon.
Figure 2
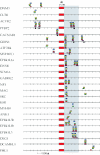
Specific location of UGCAUG hexamers near individual brain-enriched exons is highly conserved evolutionarily. The figure shows all of the brain-enriched exons for which phylogenetically conserved UGCAUG elements were identified in the flanking introns. Location of hexamers is indicated by small rectangles or circles that are color coded to indicate species. Blue, human; yellow, mouse; green, rat; red, dog; brown, chicken; and black circle, Fugu. Regulated brain-enriched exons are at the center; intron sequences to the left represent upstream sequences while those to the right represent downstream sequences.
Figure 3
UGCAUG hexamers are often situated within larger regions of conserved intron sequence. Local alignments of nucleotide sequences flanking several conserved UGCAUG elements are shown. Nucleotides shared among at least two species are shaded. Notably, UGCAUG hexamers are located within regions of conservation that may be several hundred nucleotides distal to the regulated exon. Numbers on the left indicate the distance of the UGCAUG element from the exon.
Figure 4
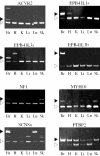
Alternative splicing of brain-enriched alternative exons is conserved in mouse. Alternative splicing of brain-enriched exons was examined by RT–PCR analysis of RNA isolated from six different mouse tissues. The figure shows polyacrylamide gel analyses indicating the amplification of brain-enriched isoforms (filled arrowheads) and non-tissue-specific isoforms (open arrowheads). The identity of the alternative exons is identified above each gel. Tissues used for PCR analysis: Br, brain; H, heart; K, kidney; Li, liver; Lu, lung and Sk, skeletal muscle.
Similar articles
- Computational analysis of candidate intron regulatory elements for tissue-specific alternative pre-mRNA splicing.
Brudno M, Gelfand MS, Spengler S, Zorn M, Dubchak I, Conboy JG. Brudno M, et al. Nucleic Acids Res. 2001 Jun 1;29(11):2338-48. doi: 10.1093/nar/29.11.2338. Nucleic Acids Res. 2001. PMID: 11376152 Free PMC article. - Variation in sequence and organization of splicing regulatory elements in vertebrate genes.
Yeo G, Hoon S, Venkatesh B, Burge CB. Yeo G, et al. Proc Natl Acad Sci U S A. 2004 Nov 2;101(44):15700-5. doi: 10.1073/pnas.0404901101. Epub 2004 Oct 25. Proc Natl Acad Sci U S A. 2004. PMID: 15505203 Free PMC article. - Tissue-dependent isoforms of mammalian Fox-1 homologs are associated with tissue-specific splicing activities.
Nakahata S, Kawamoto S. Nakahata S, et al. Nucleic Acids Res. 2005 Apr 11;33(7):2078-89. doi: 10.1093/nar/gki338. Print 2005. Nucleic Acids Res. 2005. PMID: 15824060 Free PMC article. - Fox-1 family of RNA-binding proteins.
Kuroyanagi H. Kuroyanagi H. Cell Mol Life Sci. 2009 Dec;66(24):3895-907. doi: 10.1007/s00018-009-0120-5. Cell Mol Life Sci. 2009. PMID: 19688295 Free PMC article. Review. - The impact of very short alternative splicing on protein structures and functions in the human genome.
Wen F, Li F, Xia H, Lu X, Zhang X, Li Y. Wen F, et al. Trends Genet. 2004 May;20(5):232-6. doi: 10.1016/j.tig.2004.03.005. Trends Genet. 2004. PMID: 15109776 Review. No abstract available.
Cited by
- Unusual intron conservation near tissue-regulated exons found by splicing microarrays.
Sugnet CW, Srinivasan K, Clark TA, O'Brien G, Cline MS, Wang H, Williams A, Kulp D, Blume JE, Haussler D, Ares M Jr. Sugnet CW, et al. PLoS Comput Biol. 2006 Jan;2(1):e4. doi: 10.1371/journal.pcbi.0020004. Epub 2006 Jan 20. PLoS Comput Biol. 2006. PMID: 16424921 Free PMC article. - Functional coordination of alternative splicing in the mammalian central nervous system.
Fagnani M, Barash Y, Ip JY, Misquitta C, Pan Q, Saltzman AL, Shai O, Lee L, Rozenhek A, Mohammad N, Willaime-Morawek S, Babak T, Zhang W, Hughes TR, van der Kooy D, Frey BJ, Blencowe BJ. Fagnani M, et al. Genome Biol. 2007;8(6):R108. doi: 10.1186/gb-2007-8-6-r108. Genome Biol. 2007. PMID: 17565696 Free PMC article. - ADAM15 gene structure and differential alternative exon use in human tissues.
Kleino I, Ortiz RM, Huovila AP. Kleino I, et al. BMC Mol Biol. 2007 Oct 15;8:90. doi: 10.1186/1471-2199-8-90. BMC Mol Biol. 2007. PMID: 17937806 Free PMC article. - Model-based detection of alternative splicing signals.
Barash Y, Blencowe BJ, Frey BJ. Barash Y, et al. Bioinformatics. 2010 Jun 15;26(12):i325-33. doi: 10.1093/bioinformatics/btq200. Bioinformatics. 2010. PMID: 20529924 Free PMC article. - Evolutionary divergence of exon flanks: a dissection of mutability and selection.
Xing Y, Wang Q, Lee C. Xing Y, et al. Genetics. 2006 Jul;173(3):1787-91. doi: 10.1534/genetics.106.057919. Epub 2006 May 15. Genetics. 2006. PMID: 16702427 Free PMC article.
References
- Zhu J., Mayeda A., Krainer A.R. Exon identity established through differential antagonism between exonic splicing silencer-bound hnRNP A1 and enhancer-bound SR proteins. Mol. Cell. 2001;8:1351–1361. - PubMed
- Charlet B.N., Logan P., Singh G., Cooper T.A. Dynamic antagonism between ETR-3 and PTB regulates cell type-specific alternative splicing. Mol. Cell. 2002;9:649–658. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources