Protozoan Acanthamoeba polyphaga as a potential reservoir for Campylobacter jejuni - PubMed (original) (raw)
Protozoan Acanthamoeba polyphaga as a potential reservoir for Campylobacter jejuni
Diana Axelsson-Olsson et al. Appl Environ Microbiol. 2005 Feb.
Abstract
We showed by a laboratory experiment that four different Campylobacter jejuni strains are able to infect the protozoan Acanthamoeba polyphaga. C. jejuni cells survived for longer periods when cocultured with amoebae than when grown in culture alone. The infecting C. jejuni cells aggregated in amoebic vacuoles, in which they were seen to be actively moving. Furthermore, a resuscitation of bacterial cultures that were previously negative in culturability tests was observed after reinoculation into fresh amoeba cultures. After spontaneous rupture of the amoebae, C. jejuni could be detected by microscopy and culturability tests. Our results indicate that amoebae may serve as a nonvertebrate reservoir for C. jejuni in the environment.
Figures
FIG. 1.
Experimental setup (see text for details).
FIG. 2.
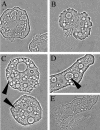
Early in the infection model, C. jejuni cells aggregated at certain positions on A. polyphaga cell walls (A and B), and after some time, live bacterial cells were observed in amoebic vacuoles (C and D). Subculturing of culture-negative samples together with fresh amoebae at 37°C resulted in lysis of the amoebae, after which live C. jejuni cells could be retrieved (E).
FIG. 3.
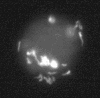
In situ hybridization with a fluorescent EUB338 probe identified the presence of intact bacterial cells within amoebic vacuoles.
FIG. 4.
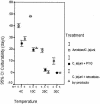
Mean percentages of retained culturability of C. jejuni grown in different media and at different temperatures. CI, confidence interval.
Similar articles
- Fate of internalized Campylobacter jejuni and Mycobacterium avium from encysted and excysted Acanthamoeba polyphaga.
Maal-Bared R, Dixon B, Axelsson-Olsson D. Maal-Bared R, et al. Exp Parasitol. 2019 Apr;199:104-110. doi: 10.1016/j.exppara.2019.03.011. Epub 2019 Mar 19. Exp Parasitol. 2019. PMID: 30902623 - Colonization of broilers by Campylobacter jejuni internalized within Acanthamoeba castellanii.
Snelling WJ, Stern NJ, Lowery CJ, Moore JE, Gibbons E, Baker C, Dooley JS. Snelling WJ, et al. Arch Microbiol. 2008 Feb;189(2):175-9. doi: 10.1007/s00203-007-0303-0. Epub 2007 Sep 19. Arch Microbiol. 2008. PMID: 17882400 - Campylobacter jejuni loss of culturability in aqueous microcosms and ability to resuscitate in a mouse model.
Baffone W, Casaroli A, Citterio B, Pierfelici L, Campana R, Vittoria E, Guaglianone E, Donelli G. Baffone W, et al. Int J Food Microbiol. 2006 Mar 1;107(1):83-91. doi: 10.1016/j.ijfoodmicro.2005.08.015. Epub 2005 Nov 14. Int J Food Microbiol. 2006. PMID: 16290304 - Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms.
Dasti JI, Tareen AM, Lugert R, Zautner AE, Gross U. Dasti JI, et al. Int J Med Microbiol. 2010 Apr;300(4):205-11. doi: 10.1016/j.ijmm.2009.07.002. Epub 2009 Aug 8. Int J Med Microbiol. 2010. PMID: 19665925 Review. - A tolerogenic mucosal immune response leads to persistent Campylobacter jejuni colonization in the chicken gut.
Hermans D, Pasmans F, Heyndrickx M, Van Immerseel F, Martel A, Van Deun K, Haesebrouck F. Hermans D, et al. Crit Rev Microbiol. 2012 Feb;38(1):17-29. doi: 10.3109/1040841X.2011.615298. Epub 2011 Oct 13. Crit Rev Microbiol. 2012. PMID: 21995731 Review.
Cited by
- Growth comparison of Acanthamoeba genotypes T3 and T4 in several culture media.
Latifi A, Salimi M. Latifi A, et al. Heliyon. 2020 Sep 14;6(9):e04805. doi: 10.1016/j.heliyon.2020.e04805. eCollection 2020 Sep. Heliyon. 2020. PMID: 32984575 Free PMC article. - Coping with Environmental Eukaryotes; Identification of Pseudomonas syringae Genes during the Interaction with Alternative Hosts or Predators.
Dorati F, Barrett GA, Sanchez-Contreras M, Arseneault T, José MS, Studholme DJ, Murillo J, Caballero P, Waterfield NR, Arnold DL, Shaw LJ, Jackson RW. Dorati F, et al. Microorganisms. 2018 Apr 21;6(2):32. doi: 10.3390/microorganisms6020032. Microorganisms. 2018. PMID: 29690522 Free PMC article. - Detection of Campylobacter spp. in water by dead-end ultrafiltration and application at farm level.
Ferrari S, Frosth S, Svensson L, Fernström LL, Skarin H, Hansson I. Ferrari S, et al. J Appl Microbiol. 2019 Oct;127(4):1270-1279. doi: 10.1111/jam.14379. Epub 2019 Jul 22. J Appl Microbiol. 2019. PMID: 31291690 Free PMC article. - Detection of Vibrio cholerae and Acanthamoeba species from same natural water samples collected from different cholera endemic areas in Sudan.
Shanan S, Abd H, Hedenström I, Saeed A, Sandström G. Shanan S, et al. BMC Res Notes. 2011 Apr 7;4:109. doi: 10.1186/1756-0500-4-109. BMC Res Notes. 2011. PMID: 21470437 Free PMC article. - Isolation of Acanthamoeba spp. from different water sources in Isfahan, central Iran, 2014.
Mohammadi Manesh R, Niyyati M, Yousefi HA, Eskandarian AA. Mohammadi Manesh R, et al. J Parasit Dis. 2016 Dec;40(4):1483-1486. doi: 10.1007/s12639-015-0716-7. Epub 2016 Jan 13. J Parasit Dis. 2016. PMID: 27876971 Free PMC article.
References
- Ahearn, D. G., and M. M. Gabriel. 1997. Contact lenses, disinfectants and acanthamoebae keratitis. Adv. Appl. Microbiol. 43:35-56. - PubMed
- Barker, J., and R. W. Brown. 1994. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology 140:1253-1259. - PubMed
- Buswell, C. M., Y. M. Herlihy, L. M. Lawrence, J. T. M. McGuiggan, P. D. Marsh, C. W. Keevil, and S. A. Leach. 1998. Extended survival and persistence of Campylobacter spp. in water and aquatic biofilms and their detection by immunofluorescent-antibody and -rRNA staining. Appl. Environ. Microbiol. 64:733-741. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources