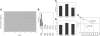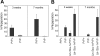Murine hematopoietic stem cells change their surface phenotype during ex vivo expansion - PubMed (original) (raw)
Murine hematopoietic stem cells change their surface phenotype during ex vivo expansion
Cheng Cheng Zhang et al. Blood. 2005.
Abstract
Ex vivo expansion of hematopoietic stem cells (HSCs) is important for many clinical applications, and knowledge of the surface phenotype of ex vivo-expanded HSCs will be critical to their purification and analysis. Here, we developed a simple culture system for bone marrow (BM) HSCs using low levels of stem cell factor (SCF), thrombopoietin (TPO), insulin-like growth factor 2 (IGF-2), and fibroblast growth factor-1 (FGF-1) in serum-free medium. As measured by competitive repopulation analyses, there was a more than 20-fold increase in numbers of long-term (LT)-HSCs after a 10-day culture of total BM cells. Culture of BM "side population" (SP) cells, a highly enriched stem cell population, for 10 days resulted in an approximate 8-fold expansion of repopulating HSCs. Similar to freshly isolated HSCs, repopulating HSCs after culture were positive for the stem cell markers Sca-1, Kit, and CD31 and receptors for IGF-2. Surprisingly, prion protein and Tie-2, which are present on freshly isolated HSCs, were not on cultured HSCs. Two other HSC markers, Endoglin and Mpl, were expressed only on a portion of cultured HSCs. Therefore, the surface phenotype of ex vivo-expanded HSCs is different from that of freshly isolated HSCs, but this plasticity of surface phenotype does not significantly alter their repopulation capability.
Figures
Figure 1.
A novel culture system of total BM cells dramatically expands HSCs. (A) Total BM cells (106) were initiated in serum-free medium with SCF, TPO, IGF-2, and FGF-1 as described in “Materials and methods,” and total cell numbers were counted at days 7, 10, 14, 21, and 28. Results from 3 independent cultures were plotted. (B) Comparison of the long-term repopulation potential of 10-day cultured and freshly isolated BM cells. We mixed 5 × 104, 1 × 104, 5000, 2500, or 1250 freshly isolated CD45.2 BM cells or 3.5 × 105, 7 × 104, 3.5 × 104, 1.75 × 104, or 9000 10-day cultured BM cells (the product of 5 × 104, 1 × 104, 5000, 2500, or 1250 initially plated CD45.2 cells, respectively) with 105 CD45.1 competitor BM cells and transplanted them into lethally irradiated recipients (n = 6 mice). In 1 case IGF-2 was not added, but the remainder of the culture conditions was unchanged. Peripheral blood cells were analyzed for the presence of CD45.2+ cells at 4 months after transplantation. Three independent experiments were performed that gave similar results. (C) Multilineage contribution of 3.5 × 105 cultured cells (derived from 5 × 104 input cells) at 4 months after transplantation (n = 6). (D) Multilineage contribution of the 3.5 × 105 cultured cells (equivalent to 5 × 104 input cells) at 4 months after transplantation of mice receiving a secondary transplant (n = 4). (E) Limiting dilution analysis of the repopulating ability of total BM cells before and after culture. Irradiated CD45.1 congenic mice were injected with 105 CD45.1 BM competitor cells and the indicated numbers of freshly isolated CD45.2 BM cells (▪ and —) or their progeny after 10 days of culture in serum-free medium with SCF, TPO, IGF-2, and FGF-1 (▿ and —). Plotted is the percentage of recipient mice containing less than 1% CD45.2 lymphoid and myeloid subpopulations in nucleated peripheral blood cells 4 months after transplantation versus the number of injected cells. The curve was anchored by the 0 cells/100% negative mice point. Error bars indicate SEM.
Figure 2.
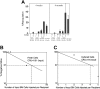
Culture dramatically increases in vivo repopulating stem-cell activity of BM SP cells. (A) Freshly isolated adult CD45.2 BM SP cells (2, 5, 25, or 100) or their progenies after 10 days of culture were transplanted (together with 1 × 105 CD45.1 competitor BM cells per mouse, n = 5-8) into CD45.1 congenic mice. Peripheral blood engraftments at 4 weeks and 4 months after transplantation are shown. Error bars indicate SEM. (B) Limiting dilution analysis of the repopulating ability of BM SP cells before culture. Irradiated CD45.1 congenic mice were injected with 105 CD45.1 BM competitor cells and 2 (n = 7 mice), 5 (n = 6), 25 (n = 8), or 100 (n = 5) freshly isolated CD45.2 BM SP cells. Similar to Figure 1E, plotted is the percentage of recipient mice containing less than 1% CD45.2 lymphoid and myeloid subpopulations in nucleated peripheral blood cells 4 months after transplantation versus the number of injected cells. Input SP cells (100) resulted in 0% of negative mice, and this data point is not plotted. (C) Limiting dilution analysis of the repopulating ability of BM SP cells after culture. The same assay as used in panel B was carried out except the progenies of the input 2 (n = 5), 5 (n = 6), 25 (n = 4), or 100 SP cells (n = 5) after 10 days of culture were injected. The cultured progeny of 25 or 100 input SP cells resulted in 0% of negative mice, and the data points are not plotted.
Figure 3.
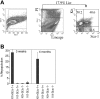
All HSCs reside in the Kit- and Sca-1–positive fraction of cultured BM cells. (A) Ten-day cultured BM cells were stained with a cocktail of biotinylated lineage-specific antibodies, followed by streptavidin-APC, anti–Sca-1–FITC, and anti-Kit–PE. Forward scatter (FSC) and side scatter (SSC) on the left plot is used to gate on hematopoietic cells. In the middle and right plots, Lin- (negative APC-stained) and propidium iodide–negative (PI-) cells were gated to show surface expression of Sca-1 and Kit. Numbers in the graph are the percentages of each cell fraction. (B) Expanded HSCs in cultured BM cells are Sca-1+Kit+. After 10 days of culture of total BM cells, 1.3 × 104 sorted CD45.2 Sca-1+Kit+, Sca-1+Kit-, Sca-1-Kit+, or Sca-1-Kit- cells were transplanted together with 2 × 105 CD45.1 competitor cells into lethally irradiated CD45.1 mice (n = 4). Peripheral blood engraftments at 3 weeks and 4 months after transplantation are shown. Error bars indicate SEM.
Figure 4.
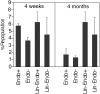
Ex vivo–expanded BM HSCs are in both Endoglin-positive and -negative fractions. After 10 days of culture of total BM cells, 6000 sorted Endoglin+ and 2.4 × 104 Endoglin- cells or 3000 Lin-Endoglin- and 3000 Lin-Endoglin+ cells were transplanted together with 2 × 105 CD45.1 competitor cells into lethally irradiated CD45.1 mice (n = 4). Shown are peripheral blood engraftment at 4 weeks (left) and 4 months (right) after transplantation. Different numbers of sorted Endoglin+ Endoglin- cells were used because after culture there were 3 times more Endoglin- cells (76% of the total) than Endoglin+ cells (26%). Error bars indicate SEM.
Figure 5.
All ex vivo–expanded BM HSCs are PrP-. (A) Either 105 PrP- or 2 × 104 PrP+ freshly isolated CD45.2 donor BM cells were mixed with 105 competitor CD45.1 cells and transplanted into lethally irradiated CD45.1 mice (n = 4). Donor CD45.2 contribution at 3 weeks (left) and 7 months (right) after transplantation are shown. (B) PrP+ and PrP- cells (5000) sorted from 10-day cultured total BM or 3000 Lin-Sca-1+PrP+ and Lin-Sca-1+PrP- cells sorted from 4-day cultured total BM cells were transplanted together with 2 × 105 CD45.1 competitor cells into lethally irradiated CD45.1 mice (n = 4). Peripheral blood engraftments at 3 weeks (left) and 7 months (right) after transplantation are shown. Error bars indicate SEM.
Figure 6.
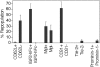
Ex vivo–expanded BM HSCs are CD62L-, IGF2-hFc+, CD31+, Tie-2-, and prominin-1-. After 10 days of culture of total BM cells, 4 × 104 sorted CD62L+ and CD62L- cells, or IGF2-hFc+ and IGF2-hFc- cells, or Mpl+ and Mpl- cells, or CD31+ and CD31- cells, or 6000 sorted Tie-2+ and Tie-2- cells, or 6000 prominin-1+ and prominin-1- cells were transplanted, respectively, together with 105 CD45.1 competitor cells into lethally irradiated CD45.1 mice (n = 4). Peripheral blood engraftments at 4 months after transplantation are shown. Error bars indicate SEM.
Similar articles
- Repopulating activity of ex vivo-expanded murine hematopoietic stem cells resides in the CD48-c-Kit+Sca-1+lineage marker- cell population.
Noda S, Horiguchi K, Ichikawa H, Miyoshi H. Noda S, et al. Stem Cells. 2008 Mar;26(3):646-55. doi: 10.1634/stemcells.2007-0623. Epub 2007 Dec 13. Stem Cells. 2008. PMID: 18079432 - Angiopoietin-like protein 3 promotes preservation of stemness during ex vivo expansion of murine hematopoietic stem cells.
Farahbakhshian E, Verstegen MM, Visser TP, Kheradmandkia S, Geerts D, Arshad S, Riaz N, Grosveld F, van Til NP, Meijerink JP. Farahbakhshian E, et al. PLoS One. 2014 Aug 29;9(8):e105642. doi: 10.1371/journal.pone.0105642. eCollection 2014. PLoS One. 2014. PMID: 25170927 Free PMC article. - Cytokine combinations on the potential for ex vivo expansion of murine hematopoietic stem cells.
Lui WC, Chan YF, Chan LC, Ng RK. Lui WC, et al. Cytokine. 2014 Aug;68(2):127-32. doi: 10.1016/j.cyto.2014.04.008. Epub 2014 May 11. Cytokine. 2014. PMID: 24825677 - Quantitative assessment of the stem cell self-renewal capacity.
Nakauchi H, Sudo K, Ema H. Nakauchi H, et al. Ann N Y Acad Sci. 2001 Jun;938:18-24; discussion 24-5. doi: 10.1111/j.1749-6632.2001.tb03570.x. Ann N Y Acad Sci. 2001. PMID: 11458506 Review. - Experimental culture conditions are critical for ex vivo expansion of hematopoietic cells.
Douay L. Douay L. J Hematother Stem Cell Res. 2001 Jun;10(3):341-6. doi: 10.1089/152581601750288948. J Hematother Stem Cell Res. 2001. PMID: 11454309 Review.
Cited by
- Alpharetroviral vector-mediated gene therapy for X-CGD: functional correction and lack of aberrant splicing.
Kaufmann KB, Brendel C, Suerth JD, Mueller-Kuller U, Chen-Wichmann L, Schwäble J, Pahujani S, Kunkel H, Schambach A, Baum C, Grez M. Kaufmann KB, et al. Mol Ther. 2013 Mar;21(3):648-61. doi: 10.1038/mt.2012.249. Epub 2012 Dec 4. Mol Ther. 2013. PMID: 23207695 Free PMC article. - Competitive Transplants to Evaluate Hematopoietic Stem Cell Fitness.
Kwarteng EO, Heinonen KM. Kwarteng EO, et al. J Vis Exp. 2016 Aug 31;(114):54345. doi: 10.3791/54345. J Vis Exp. 2016. PMID: 27684883 Free PMC article. - The Periostin/Integrin-αv Axis Regulates the Size of Hematopoietic Stem Cell Pool in the Fetal Liver.
Biswas A, Roy IM, Babu PC, Manesia J, Schouteden S, Vijayakurup V, Anto RJ, Huelsken J, Lacy-Hulbert A, Verfaillie CM, Khurana S. Biswas A, et al. Stem Cell Reports. 2020 Aug 11;15(2):340-357. doi: 10.1016/j.stemcr.2020.06.022. Epub 2020 Jul 30. Stem Cell Reports. 2020. PMID: 32735820 Free PMC article. - High-resolution video monitoring of hematopoietic stem cells cultured in single-cell arrays identifies new features of self-renewal.
Dykstra B, Ramunas J, Kent D, McCaffrey L, Szumsky E, Kelly L, Farn K, Blaylock A, Eaves C, Jervis E. Dykstra B, et al. Proc Natl Acad Sci U S A. 2006 May 23;103(21):8185-90. doi: 10.1073/pnas.0602548103. Epub 2006 May 15. Proc Natl Acad Sci U S A. 2006. PMID: 16702542 Free PMC article. - Clonal interrogation of stem cells.
Hope K, Bhatia M. Hope K, et al. Nat Methods. 2011 Apr;8(4 Suppl):S36-40. doi: 10.1038/nmeth.1590. Nat Methods. 2011. PMID: 21451511 Review.
References
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241: 58-62. - PubMed
- Jordan CT, McKearn JP, Lemischka IR. Cellular and developmental properties of fetal hematopoietic stem cells. Cell. 1990;61: 953-963. - PubMed
- Rebel VI, Miller CL, Thornbury GR, Dragowska WH, Eaves CJ, Lansdorp PM. A comparison of long-term repopulating hematopoietic stem cells in fetal liver and adult bone marrow from the mouse. Exp Hematol. 1996;24: 638-648. - PubMed
- Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273: 242-245. - PubMed
- Solar GP, Kerr WG, Zeigler FC, et al. Role of c-mpl in early hematopoiesis. Blood. 1998;92: 4-10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous