Apoptosis-inducing factor is a key factor in neuronal cell death propagated by BAX-dependent and BAX-independent mechanisms - PubMed (original) (raw)
Apoptosis-inducing factor is a key factor in neuronal cell death propagated by BAX-dependent and BAX-independent mechanisms
Eric C C Cheung et al. J Neurosci. 2005.
Abstract
Mitochondria release proteins that propagate both caspase-dependent and caspase-independent cell death pathways. AIF (apoptosis-inducing factor) is an important caspase-independent death regulator in multiple neuronal injury pathways. Presently, there is considerable controversy as to whether AIF is neuroprotective or proapoptotic in neuronal injury, such as oxidative stress or excitotoxicity. To evaluate the role of AIF in BAX-dependent (DNA damage induced) and BAX-independent (excitotoxic) neuronal death, we used Harlequin (Hq) mice, which are hypomorphic for AIF. Neurons carrying double mutations for Hq/Apaf1-/- (apoptosis proteases-activating factor) are impaired in both caspase-dependent and AIF-mediated mitochondrial cell death pathways. These mutant cells exhibit extended neuroprotection against DNA damage, as well as glutamate-induced excitotoxicity. Specifically, AIF is involved in NMDA- and kainic acid- but not AMPA-induced excitotoxicity. In vivo excitotoxic studies using kainic acid-induced seizure showed that Hq mice had significantly less hippocampal damage than wild-type littermates. Our results demonstrate an important role for AIF in both BAX-dependent and BAX-independent mechanisms of neuronal injury.
Figures
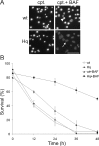
Figure 1.
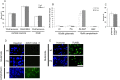
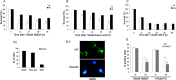
Reduced AIF in Hq mice protects neurons from camptothecin-induced neuronal cell death in the absence of caspase activity. Cortical neurons from wild-type (wt) and Hq littermates were treated with camptothecin (cpt.) (10 μ
m
), with or without BAF (10 μ
m
). A, Photomicrographs of neurons after 36 h of treatment. Scale bar, 50 μm. Neurons were stained with Hoechst 33258 to assess nuclear morphology. The arrow indicates morphologically healthy nuclei, and the arrowhead indicates pyknotic condensed nuclei that denote cell death. B, Quantitative analysis of neuronal survival rate at the indicated time points after camptothecin treatment, with or without BAF. Survival as determined by nuclear morphology is reported as a percentage of live cells over the total number of cells (n = 3).
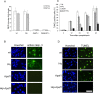
Figure 2.
Caspase activation and DNA fragmentation induced by camptothecin is reduced in _Apaf1_-/- and _Hq/Apaf1_-/- neurons. Cortical neurons cultured from wild-type (wt), _Hq, Apaf1_-/-, and _Hq/Apaf1_-/- littermates were treated with camptothecin (10 μ
m
). A, Caspase activity was measured by DEVD-AFC (_N_-acetyl-Asp-Glu-Val-Asp-(7-amino-4-trifluoromethyl-coumarin)) cleavage (n = 3). B, Immunocytochemistry of active caspase 3 and nuclear staining by Hoechst at 24 h. C, Quantification of TUNEL staining (n = 3). D, TUNEL staining and nuclear staining by Hoechst at 24 h. Scale bar, 50 μm.
Figure 3.
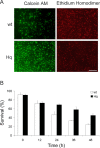
Inhibition of caspases and AIF in _Hq/Apaf1_-/- double mutants increases the survival of cortical neurons against camptothecin-induced cell death. Cortical neurons from wild-type (wt), _Apaf1_-/-, Hq, and _Hq/Apaf1_-/- littermates were treated with camptothecin (10 μ
m
). Neuronal survival was measured by live/dead assay. Live cells exhibit staining for calcein-AM activity (green fluorescence), whereas dead cells stain positive for ethidium homodimer (red fluorescence). A, Neurons after 36 h of camptothecin treatment with live/dead staining. Scale bar, 100 μm. B, Quantitative analysis of survival rate measured by live/dead assay after camptothecin treatment (n = 3). C, Quantitative analysis of propidium iodide-positive (PI+) and TUNEL-positive (TUNEL+) neurons 48 h after camptothecin treatment (n = 3). *p < 0.01 compared with PI+.
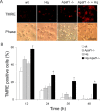
Figure 4.
A higher proportion of _Hq/Apaf1_-/- double-mutant neurons retain mitochondrial membrane potential after camptothecin treatment. Cortical neurons from wild-type (wt), _Apaf1_-/-, Hq, and _Hq/Apaf1_-/- littermates were treated with camptothecin (10 μ
m
), and mitochondrial membrane potential was assessed by TMRE, which only incorporates into mitochondria with intact membrane potential. A, Neurons 36 h after camptothecin treatment. Scale bar, 50 μm. Phase, Phase contrast microscopy. B, Quantitative analysis of neurons with positive TMRE staining, which indicates intact mitochondrial membrane potential at the indicated times. TMRE-positive cells are reported as a percentage of stained cells over the total number of cells (n = 3).
Figure 5.
Excitotoxicity-induced neuronal cell death is BAX and caspase independent. A, Quantification of survival by MTT assay in cortical neurons and CGNs from BAX-deficient and wild-type (wt) littermates after 24 h of treatment with 100 μ
m
glutamate and 200 μ
m
NMDA for cortical neurons and 100 μ
m
glutamate for CGNs (n = 4). B, Caspase 3 activity is measured by DEVD-AFC cleavage in wild-type and Hq cortical neurons after 24 h of treatment with 100 μ
m
glutamate. Camptothecin-treated wild-type neurons with or without BAF are used as positive and negative controls, respectively (n = 3). C, MTT assay for wild-type neurons treated with 100 μ
m
glutamate in the presence or absence of 50 μ
m
BAF (n = 3). Note that BAF does not provide protection after exposure to glutamate. D, Active caspase 3 immunohistochemistry 24 h after 100 μ
m
glutamate treatment and camptothecin-treated wild-type cells were used as a control. E, TUNEL staining of cortical neurons 24 h after 100 μ
m
glutamate treatment, indicating DNA fragmentation in the absence of caspase. Scale bar, 50 μm.
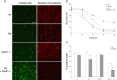
Figure 6.
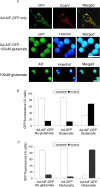
AIF translocates to the nucleus during glutamate-induced cell death. A, Cortical neurons from wild-type mice were infected with recombinant adenoviral vector (Ad) containing GFP-tagged AIF and were treated with glutamate (100 μ
m
) for 1 h. After 24 h, cells were fixed and stained with Hoechst for nucleus and CoxVI antibody for mitochondria, and GFP fluorescence was observed. Translocation of endogenous AIF was also assessed using AIF antibody and Hoechst staining. Scale bar, 10 μm. B, Quantitative analysis of GFP fluorescence in the nucleus in cortical neurons (B) and CGNs (C) (n = 3).
Figure 7.
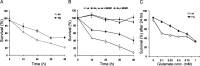
Hq cortical neurons are more resistant to excitotoxicity induced by glutamate. Cortical neurons (14 DIV) from wild-type (wt) and Hq littermates were treated with glutamate (100 μ
m
) for 1 h. A, Quantitative analysis of neuronal survival rate by live/dead assay at the indicated time points after glutamate treatment (n = 3). B, Quantitative analysis of neuronal survival rate by nuclear morphology revealed by Hoechst staining at the indicated time points after glutamate treatment (n = 3). Survival is reported as a percentage of cells with morphologically healthy nuclei over the total number of cells. C, Quantitative analysis of cortical neuron survival with increasing glutamate concentration after 24 h (n = 3).
Figure 8.
Hq CGNs are more resistant to excitotoxicity induced by glutamate. CGNs (14 DIV) from wild-type (wt) and Hq littermates were treated with glutamate (100 μ
m
) for 1 h. Neuronal survival was determined with live/dead assay. A, Photomicrographs of neurons 36 h after glutamate treatment. Scale bar, 250 μm. B, Quantitative analysis of survival rate at the indicated time points after glutamate treatment (n = 3).
Figure 9.
Hq cortical neurons are more resistant to excitotoxicity induced by NMDA and kainate but not AMPA. Cortical neurons (14 DIV) from wild-type (wt) and Hq littermates were treated with 100 μ
m
NMDA (A), 100 μ
m
kainic acid (B), and 100 μ
m
AMPA (C) for 1 h with the appropriate coagonists, desensitization blockers, and specific receptor blockers (see Materials and Methods) (n = 3). Survival was determined by nuclear morphology using Hoechst stain. Di, Nuclear translocation of AIF assessed by AIF immunohistochemistry after NMDA, kainate, and AMPA treatment (n = 3). Dii, Photomicrographs of AIF immunohistochemistry show the lack of nuclear translocation after AMPA treatment. Scale bar, 10 μm. E, Quantitative analysis of PI-positive (PI+) and TUNEL-positive (TUNEL+) neurons 36 h after NMDA and kainate treatment (n = 3). *p < 0.01 compared with wild-type (A, B) or PI positive (C).
Figure 10.
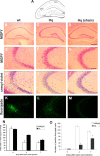
Hq neurons are more resistant to excitotoxicity in vivo induced by kainic acid seizure in adult mice. Animals were injected with kainic acid (30 mg/kg) and killed 4 or 7 d afterward. A, Diagram of coronal section of hippocampus. The box indicates the CA3ab region. DG, Dentate gyrus. B-D, MGPY staining was used to assess viability. Scale bar, 500 μm. E-G, MGPY staining at higher magnification. Scale bar, 100 μm. H-J, Cresyl violet staining. K-M, FluoroJade staining was used to assess cell death. Sham is Hq mice. N, Quantification of viable cells per 0.1 mm2 in CA3ab area by MGPY stain (n = 3). *p < 0.01 compared with wild type. O, Quantification of cell death in the CA3ab area by FluoroJade staining (n = 3). *p < 0.05 compared with wild type.
Similar articles
- Apoptosis-inducing factor substitutes for caspase executioners in NMDA-triggered excitotoxic neuronal death.
Wang H, Yu SW, Koh DW, Lew J, Coombs C, Bowers W, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Wang H, et al. J Neurosci. 2004 Dec 1;24(48):10963-73. doi: 10.1523/JNEUROSCI.3461-04.2004. J Neurosci. 2004. PMID: 15574746 Free PMC article. - Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death.
Cregan SP, Fortin A, MacLaurin JG, Callaghan SM, Cecconi F, Yu SW, Dawson TM, Dawson VL, Park DS, Kroemer G, Slack RS. Cregan SP, et al. J Cell Biol. 2002 Aug 5;158(3):507-17. doi: 10.1083/jcb.200202130. Epub 2002 Jul 29. J Cell Biol. 2002. PMID: 12147675 Free PMC article. - Hierarchical recruitment by AMPA but not staurosporine of pro-apoptotic mitochondrial signaling in cultured cortical neurons: evidence for caspase-dependent/independent cross-talk.
Beart PM, Lim ML, Chen B, Diwakarla S, Mercer LD, Cheung NS, Nagley P. Beart PM, et al. J Neurochem. 2007 Dec;103(6):2408-27. doi: 10.1111/j.1471-4159.2007.04937.x. Epub 2007 Sep 20. J Neurochem. 2007. PMID: 17887970 - Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria.
Candé C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N, Kroemer G. Candé C, et al. Biochimie. 2002 Feb-Mar;84(2-3):215-22. doi: 10.1016/s0300-9084(02)01374-3. Biochimie. 2002. PMID: 12022952 Review. - Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death?
Candé C, Cecconi F, Dessen P, Kroemer G. Candé C, et al. J Cell Sci. 2002 Dec 15;115(Pt 24):4727-34. doi: 10.1242/jcs.00210. J Cell Sci. 2002. PMID: 12432061 Review.
Cited by
- Protection by Nano-Encapsulated Bacoside A and Bacopaside I in Seizure Alleviation and Improvement in Sleep- In Vitro and In Vivo Evidences.
C Sekhar V, Gulia KK, Deepti A, Chakrapani PSB, Baby S, Viswanathan G. C Sekhar V, et al. Mol Neurobiol. 2024 Jun;61(6):3296-3313. doi: 10.1007/s12035-023-03741-w. Epub 2023 Nov 21. Mol Neurobiol. 2024. PMID: 37987958 - Enhanced cell death in MeCP2 null cerebellar granule neurons exposed to excitotoxicity and hypoxia.
Russell JC, Blue ME, Johnston MV, Naidu S, Hossain MA. Russell JC, et al. Neuroscience. 2007 Dec 12;150(3):563-74. doi: 10.1016/j.neuroscience.2007.09.076. Epub 2007 Oct 11. Neuroscience. 2007. PMID: 17997046 Free PMC article. - Dissociating the dual roles of apoptosis-inducing factor in maintaining mitochondrial structure and apoptosis.
Cheung EC, Joza N, Steenaart NA, McClellan KA, Neuspiel M, McNamara S, MacLaurin JG, Rippstein P, Park DS, Shore GC, McBride HM, Penninger JM, Slack RS. Cheung EC, et al. EMBO J. 2006 Sep 6;25(17):4061-73. doi: 10.1038/sj.emboj.7601276. Epub 2006 Aug 17. EMBO J. 2006. PMID: 16917506 Free PMC article. - Apoptosis, Bcl-2 family proteins and caspases: the ABCs of seizure-damage and epileptogenesis?
Engel T, Henshall DC. Engel T, et al. Int J Physiol Pathophysiol Pharmacol. 2009 Mar 30;1(2):97-115. Int J Physiol Pathophysiol Pharmacol. 2009. PMID: 21383882 Free PMC article. - Inhibition of nuclear translocation of apoptosis-inducing factor is an essential mechanism of the neuroprotective activity of pigment epithelium-derived factor in a rat model of retinal degeneration.
Murakami Y, Ikeda Y, Yonemitsu Y, Onimaru M, Nakagawa K, Kohno R, Miyazaki M, Hisatomi T, Nakamura M, Yabe T, Hasegawa M, Ishibashi T, Sueishi K. Murakami Y, et al. Am J Pathol. 2008 Nov;173(5):1326-38. doi: 10.2353/ajpath.2008.080466. Epub 2008 Oct 9. Am J Pathol. 2008. PMID: 18845835 Free PMC article.
References
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M (2002) Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science 298: 846-850. - PubMed
- Al-Hazzaa AA, Bowen ID (1998) Improved cytochemical methods for demonstrating cell death using LR White as an embedding medium. Histochem J 30: 897-902. - PubMed
- Arundine M, Tymianski M (2003) Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 34: 325-337. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials