Glutamine-dependent inhibition of pial arteriolar dilation to acetylcholine with and without hyperammonemia in the rat - PubMed (original) (raw)
Glutamine-dependent inhibition of pial arteriolar dilation to acetylcholine with and without hyperammonemia in the rat
Tetsu Kawaguchi et al. Am J Physiol Regul Integr Comp Physiol. 2005 Jun.
Abstract
Glutamine has been shown to influence endothelial-dependent relaxation and nitric oxide production in vitro, possibly by limiting arginine availability, but its effects in vivo have not been well studied. Hyperammonemia is a pathophysiological condition in which glutamine is elevated and contributes to depressed CO(2) reactivity of cerebral arterioles. We tested the hypothesis that acute hyperammonemia decreases pial arteriolar dilation to acetylcholine in vivo and that this decrease could be prevented by inhibiting glutamine synthetase with L-methionine-S-sulfoximine (MSO) or by intravenous infusion of L-arginine. Pial arteriolar diameter responses to topical superfusion of acetylcholine were measured in anesthetized rats before and at 6 h of infusion of either sodium or ammonium acetate. Ammonium acetate infusion increased plasma ammonia concentration from approximately 30 to approximately 600 microM and increased cerebral glutamine concentration fourfold. Arteriolar dilation to acetylcholine was intact after infusion of sodium acetate in groups pretreated with vehicle or with MSO plus methionine, which was coadministered to prevent MSO-induced seizures. In contrast, dilation in response to acetylcholine was completely blocked in hyperammonemic groups pretreated with vehicle or methionine alone. However, MSO plus methionine administration before hyperammonemia, which maintained cerebral glutamine concentration at control values, preserved acetylcholine dilation. Intravenous infusion of L-arginine during the last 2 h of the ammonium acetate infusion partially restored dilation to acetylcholine without reducing cerebral glutamine accumulation. Superfusion of 1 or 2 mM L-glutamine through the cranial window for 1 h in the absence of hyperammonemia attenuated acetylcholine dilation but had no effect on endothelial-independent dilation to nitroprusside. We conclude that 1) hyperammonemia reduces acetylcholine-evoked dilation in cerebral arterioles, 2) this reduction depends on increased glutamine rather than ammonium ions, and 3) increasing arginine partially overcomes the inhibitory effect of glutamine.
Figures
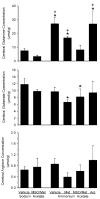
Fig. 1
Cerebral tissue concentration (±SD) of glutamine (top), glutamate (middle), and arginine (bottom) after a 6-h infusion of sodium acetate in groups pretreated with vehicle (n = 5) and L-methionine-_S_-sulfoximine (MSO) + L-methionine (Met; n = 6) and after a 6-h infusion of ammonium acetate in groups pretreated with vehicle (n = 5), Met (n = 6), MSO/Met (n = 6), and L-arginine (Arg; n = 5). *P < 0.05 from vehicle-sodium acetate group; +P < 0.05 from MSO/Met-ammonium acetate group. Arginine concentration in the L-arginine group was greater than in the Met-ammonium acetate group but was not significant from the other groups.
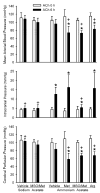
Fig. 2
Mean arterial blood pressure (top), intracranial pressure (middle), and cerebral perfusion pressure (bottom) (±SD) during ACh superfusion before (0 h) and at 6 h of infusion of sodium acetate in groups pretreated with vehicle (n = 8) and MSO/Met (n = 8) and after 6 h of infusion of ammonium acetate in groups pretreated with vehicle (n = 8), Met (n = 6), MSO/Met (n = 8), and Arg (n = 8). *P < 0.05 from 0-h value within group; +P < 0.05 from vehicle-ammonium acetate group at same time point.
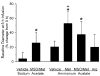
Fig. 3
Percent change in baseline pial arteriolar diameter (3SD) after 6 h of infusion of sodium acetate in groups pretreated with vehicle (n = 8) and MSO/Met (n = 8) and after 6 h infusion of ammonium acetate in groups pretreated with vehicle (n = 8), Met (n = 6), MSO/Met (n = 8), and Arg (n = 8). *P < 0.05 from vehicle-sodium acetate group; +P < 0.05 from vehicle-ammonium acetate group.
Fig. 4
Percent change in pial arteriolar diameter (±SD) during 30 M ACh superfusion before (0 h) and at 6 h of infusion of sodium acetate in groups pretreated with vehicle (n = 8) and MSO/Met (n = 8) and after 6 h of infusion of ammonium acetate in groups pretreated with vehicle (n = 8), Met (n = 6), MSO/Met (n = 8), and Arg (n = 8). *P < 0.05 from 0-h value within group;+P < 0.05 from vehicle-ammonium acetate group at same time point.
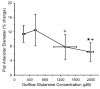
Fig. 5
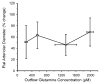
Percent change in pial arteriolar diameter (±SD) during 10 μM ACh superfusion vs. glutamine concentration in outflow of cranial window after 1 h of superfusion with 0 (n = 7), 0.3 (n = 6), 1 (n = 7), and 2 mM (n = 7) glutamine in inflow. *P < 0.05 from diameter at 0 mM inflow glutamine; P < 0.05 from diameter at 0.3 mM inflow glutamine.
Fig. 6
Percent change in pial arteriolar diameter (±SD) during 3 μM sodium nitroprusside superfusion vs. glutamine concentration in outflow of cranial window after 1 h of superfusion with 0 (n = 7), 0.3 (n = 6), 1 (n = 7), and 2 mM (n = 7) glutamine in inflow. There was no significant effect of glutamine.
Similar articles
- Impaired pial arteriolar reactivity to hypercapnia during hyperammonemia depends on glutamine synthesis.
Hirata T, Koehler RC, Kawaguchi T, Brusilow SW, Traystman RJ. Hirata T, et al. Stroke. 1996 Apr;27(4):729-36. doi: 10.1161/01.str.27.4.729. Stroke. 1996. PMID: 8614939 - Preserved hypocapnic pial arteriolar constriction during hyperammonemia by glutamine synthetase inhibition.
Hirata T, Kawaguchi T, Brusilow SW, Traystman RJ, Koehler RC. Hirata T, et al. Am J Physiol. 1999 Feb;276(2):H456-63. doi: 10.1152/ajpheart.1999.276.2.H456. Am J Physiol. 1999. PMID: 9950845 - Interaction of glutamine and arginine on cerebrovascular reactivity to hypercapnia.
Okada T, Watanabe Y, Brusilow SW, Traystman RJ, Koehler RC. Okada T, et al. Am J Physiol Heart Circ Physiol. 2000 May;278(5):H1577-84. doi: 10.1152/ajpheart.2000.278.5.H1577. Am J Physiol Heart Circ Physiol. 2000. PMID: 10775136 - Possible treatment of end-stage hyperammonemic encephalopathy by inhibition of glutamine synthetase.
Cooper AJ. Cooper AJ. Metab Brain Dis. 2013 Jun;28(2):119-25. doi: 10.1007/s11011-012-9338-2. Epub 2012 Oct 13. Metab Brain Dis. 2013. PMID: 23065027 Free PMC article. Review. - Cerebral ammonia metabolism in normal and hyperammonemic rats.
Cooper AJ, Lai JC. Cooper AJ, et al. Neurochem Pathol. 1987 Feb-Apr;6(1-2):67-95. doi: 10.1007/BF02833601. Neurochem Pathol. 1987. PMID: 2888066 Review.
Cited by
- Effects of L-glutamine supplementation on maternal and fetal hemodynamics in gestating ewes exposed to alcohol.
Sawant OB, Ramadoss J, Hankins GD, Wu G, Washburn SE. Sawant OB, et al. Amino Acids. 2014 Aug;46(8):1981-96. doi: 10.1007/s00726-014-1751-x. Epub 2014 May 9. Amino Acids. 2014. PMID: 24810329 Free PMC article. - A chronic scheme of cranial window preparation to study pial vascular reactivity in murine cerebral malaria.
Ong PK, Meays D, Frangos JA, Carvalho LJ. Ong PK, et al. Microcirculation. 2013 Jul;20(5):394-404. doi: 10.1111/micc.12034. Microcirculation. 2013. PMID: 23279271 Free PMC article. - Astrocyte glutamine synthetase: importance in hyperammonemic syndromes and potential target for therapy.
Brusilow SW, Koehler RC, Traystman RJ, Cooper AJ. Brusilow SW, et al. Neurotherapeutics. 2010 Oct;7(4):452-70. doi: 10.1016/j.nurt.2010.05.015. Neurotherapeutics. 2010. PMID: 20880508 Free PMC article. Review. - Role of astrocytes in cerebrovascular regulation.
Koehler RC, Gebremedhin D, Harder DR. Koehler RC, et al. J Appl Physiol (1985). 2006 Jan;100(1):307-17. doi: 10.1152/japplphysiol.00938.2005. J Appl Physiol (1985). 2006. PMID: 16357084 Free PMC article. Review. - Acute Hyperammonemia Induces NMDA-Mediated Hypophosphorylation of Intermediate Filaments Through PP1 and PP2B in Cerebral Cortex of Young Rats.
Carvalho RV, da Silva Ferreira F, Heimfarth L, Pierozan P, Fernandes C, Pessoa-Pureur R. Carvalho RV, et al. Neurotox Res. 2016 Aug;30(2):138-49. doi: 10.1007/s12640-016-9607-7. Epub 2016 Mar 2. Neurotox Res. 2016. PMID: 26936604
References
- Barzilay Z, Britten AG, Koehler RC, Dean JM, Traystman RJ. Interaction of CO2 and ammonia on cerebral blood flow and O2 consumption in dogs. Am J Physiol Heart Circ Physiol. 1985;248:H500–H507. - PubMed
- Brusilow SW. Determination of urine orotate and orotidine and plasma ammonium. In: Hommes FA, editor. Techniques in Diagnostic Human Biochemical Genetics: A Laboratory Manual. New York: Wiley-Liss; 1991. pp. 345–357.
- Buga GM, Singh R, Pervin S, Rogers NE, Schmitz DA, Jenkinson CP, Cederbaum SD, Ignarro LJ. Arginase activity in endothelial cells: inhibition by NG-hydroxy-L-arginine during high-output NO production. Am J Physiol Heart Circ Physiol. 1996;271:H1988–H1998. - PubMed
- Chen FY, Lee TJF. Arginine synthesis from citrulline in perivascular nerves of cerebral artery. J Pharmacol Exp Ther. 1995;273:895–901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources