Neurochemical mechanisms underlying alcohol withdrawal - PubMed (original) (raw)
Review
Neurochemical mechanisms underlying alcohol withdrawal
J Littleton. Alcohol Health Res World. 1998.
Abstract
More than 50 years ago, C.K. Himmelsbach first suggested that physiological mechanisms responsible for maintaining a stable state of equilibrium (i.e., homeostasis) in the patient's body and brain are responsible for drug tolerance and the drug withdrawal syndrome. In the latter case, he suggested that the absence of the drug leaves these same homeostatic mechanisms exposed, leading to the withdrawal syndrome. This theory provides the framework for a majority of neurochemical investigations of the adaptations that occur in alcohol dependence and how these adaptations may precipitate withdrawal. This article examines the Himmelsbach theory and its application to alcohol withdrawal; reviews the animal models being used to study withdrawal; and looks at the postulated neuroadaptations in three systems-the gamma-aminobutyric acid (GABA) neurotransmitter system, the glutamate neurotransmitter system, and the calcium channel system that regulates various processes inside neurons. The role of these neuroadaptations in withdrawal and the clinical implications of this research also are considered.
Figures
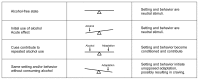
The seesaw analogy for neuroadaptation applied to conditioning and craving. The top row shows brain neurochemistry in an alcohol-free state. At this time the environment in which alcohol is consumed (i.e., the setting) and the behavior involved in drinking are neutral stimuli; that is, they have no association with drinking and no special impact on the brain. The same is true of the initial use of alcohol (second row), in which the brain’s neurochemistry is unbalanced by alcohol’s actions. If the drug is taken repeatedly in the same setting and in the same way, however, these stimuli become capable of eliciting neuroadaptations similar to those elicited by alcohol itself (third row). Exposure to such conditioned stimuli in the absence of alcohol may thus lead to unopposed neuroadaptation, which potentially may be expressed as a craving for alcohol.
Figure 1
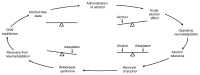
Pictorial representation of the Himmelsbach hypothesis as it applies to alcohol use. The balanced seesaw on the upper left side of the cycle represents brain neurochemistry in an alcohol-free state (i.e., before the brain has been exposed to alcohol). Consuming alcohol initially unbalances brain chemistry to produce the acute effects associated with alcohol use (e.g., sedation and incoordination). The brain then responds to this disruption by inducing an opposing chemical adaptation that tends to restore the neurochemical balance. At this stage, the effects of a given dose of alcohol are diminished (i.e., tolerance exists). If alcohol is removed, the adaptation is exposed, unbalancing the brain’s neurochemistry in the opposite direction. The result is a withdrawal syndrome that includes signs and symptoms (e.g., agitation and seizures) that are opposite to alcohol’s initial effects. These disturbances will continue until the adaptation can be removed from the brain (or until alcohol is consumed again), restoring equilibrium. 1CNS = central nevous system.
Figure 2
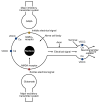
Schematic representation of some of the major neurochemical systems affected by alcohol. Nerve cells (i.e., neurons) convert chemical messages received at the cell body (at left in this simplified neuron) into an electrical signal that is conducted along the axon to the terminal (at right). At the terminal, the electrical signal is converted back into a chemical message (i.e., a neurotransmitter) that is released from the terminal and carries the information to the next neuron in the circuit. Alcohol increases (i.e., potentiates) the effects of the major inhibitory neurotransmitter in the effects tend to inhibit electrical signaling through the neuron. Alcohol further decreases electrical activity by inhibiting the major excitatory neurotransmitter, glutamate, particularly at a glutamate-receptor protein called the _N_-methyl-
d
-aspartate (NMDA) receptor. By inhibiting glutamate at the NMDA receptor, alcohol slows the flow of calcium (Ca) into cells. Regulation of the cell’s calcium balance is essential for normal cell function. In addition to its effects at the NMDA receptor, alcohol can alter the flow of calcium through voltage-operated calcium channels (VOCC’s) at the cell body as well as at the terminal, where calcium is necessary for neurotransmitter release.
Figure 3
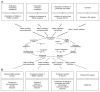
Effects of alcohol and possible relevance to dependence and withdrawal. (A) The major acute actions of alcohol and their possible relation to the behavioral consequences of drinking alcohol are shown in relation to the Himmelsbach glutamate at _N_-methyl-
d
-aspartate (NMDA) receptors, and inhibition of voltage-operated calcium channels (VOCC’s) may underlie the relaxation, intoxication, anesthesia, and amnesia caused by alcohol. Alcohol also increases release of the neurotransmitter dopamine (DA) in a specific area of the brain, the nucleus accumbens. This action of alcohol is not very well understood but may play an important role in the rewarding effects of drinking, such as euphoria. (B) Examples of the adaptive changes thought to oppose the acute effects of alcohol. The bottom panels show possible consequences of these adaptations during withdrawal. For example, the adaptive changes in GABA receptor proteins caused by alcohol may make benzodiazepine tranquilizers, which also act on GABA receptors, less effective (i.e., may produce tolerance). A reduction in DA release in the nucleus accumbens may accompany alcohol withdrawal and may contribute to depression, anxiety, and emotional discomfort (i.e., dysphoria), perhaps leading an alcoholic to resume his or her drinking. 1CNS = central nevous system.
Similar articles
- Neurochemical mechanisms of alcohol withdrawal.
Becker HC, Mulholland PJ. Becker HC, et al. Handb Clin Neurol. 2014;125:133-56. doi: 10.1016/B978-0-444-62619-6.00009-4. Handb Clin Neurol. 2014. PMID: 25307573 Free PMC article. Review. - How Imaging Glutamate, γ-Aminobutyric Acid, and Dopamine Can Inform the Clinical Treatment of Alcohol Dependence and Withdrawal.
Hillmer AT, Mason GF, Fucito LM, O'Malley SS, Cosgrove KP. Hillmer AT, et al. Alcohol Clin Exp Res. 2015 Dec;39(12):2268-82. doi: 10.1111/acer.12893. Epub 2015 Oct 28. Alcohol Clin Exp Res. 2015. PMID: 26510169 Free PMC article. Review. - Imbalance between neuroexcitatory and neuroinhibitory amino acids causes craving for ethanol.
De Witte P. De Witte P. Addict Behav. 2004 Sep;29(7):1325-39. doi: 10.1016/j.addbeh.2004.06.020. Addict Behav. 2004. PMID: 15345268 - Differential adaptations in GABAergic and glutamatergic systems during ethanol withdrawal in male and female rats.
Alele PE, Devaud LL. Alele PE, et al. Alcohol Clin Exp Res. 2005 Jun;29(6):1027-34. doi: 10.1097/01.alc.0000167743.96121.40. Alcohol Clin Exp Res. 2005. PMID: 15976529 - Alteration of glutamate/GABA balance during acute alcohol withdrawal in emergency department: a prospective analysis.
Brousse G, Arnaud B, Vorspan F, Richard D, Dissard A, Dubois M, Pic D, Geneste J, Xavier L, Authier N, Sapin V, Llorca PM, De Chazeron I, Minet-Quinard R, Schmidt J. Brousse G, et al. Alcohol Alcohol. 2012 Sep-Oct;47(5):501-8. doi: 10.1093/alcalc/ags078. Epub 2012 Jul 11. Alcohol Alcohol. 2012. PMID: 22791370
Cited by
- Role of Hippocampal Neurogenesis in Alcohol Withdrawal Seizures.
Basu S, Suh H. Basu S, et al. Brain Plast. 2020 Dec 29;6(1):27-39. doi: 10.3233/BPL-200114. Brain Plast. 2020. PMID: 33680844 Free PMC article. Review. - Will This Hospitalized Patient Develop Severe Alcohol Withdrawal Syndrome?: The Rational Clinical Examination Systematic Review.
Wood E, Albarqouni L, Tkachuk S, Green CJ, Ahamad K, Nolan S, McLean M, Klimas J. Wood E, et al. JAMA. 2018 Aug 28;320(8):825-833. doi: 10.1001/jama.2018.10574. JAMA. 2018. PMID: 30167704 Free PMC article. Review. - Alcohol's effects on sleep in alcoholics.
Brower KJ. Brower KJ. Alcohol Res Health. 2001;25(2):110-25. Alcohol Res Health. 2001. PMID: 11584550 Free PMC article. Review. - MK-801 administration during neonatal ethanol withdrawal attenuates interpositus cell loss and juvenile eyeblink conditioning deficits.
Young BW, Sengelaub DR, Steinmetz JE. Young BW, et al. Alcohol. 2010 Jun;44(4):359-69. doi: 10.1016/j.alcohol.2009.12.002. Epub 2010 Jul 3. Alcohol. 2010. PMID: 20598489 Free PMC article. - Substantia nigra pars reticulata is crucially involved in barbiturate and ethanol withdrawal in mice.
Chen G, Kozell LB, Buck KJ. Chen G, et al. Behav Brain Res. 2011 Mar 17;218(1):152-7. doi: 10.1016/j.bbr.2010.10.025. Epub 2010 Oct 23. Behav Brain Res. 2011. PMID: 20974184 Free PMC article.
References
- Connors GJ, Maisto SA, Donovan DM. Conceptualizations of relapse: A summary of psychological and psychobiological models. Addiction. 1996;91(Suppl):S5–S13. - PubMed
- Spanagel R, Ziegelgansberger W. Anti-craving compounds for ethanol: New pharmacological tools to study addictive processes. Trends in Pharmacological Science. 1997;18:54–59. - PubMed
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: APA; 1994.
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. British Journal of Psychiatry. 1978;133:1–14. - PubMed
- Becker HC, Littleton JM. The alcohol withdrawal “kindling” phenomenon: Clinical and experimental findings. Alcoholism: Clinical and Experimental Research. 1996;20:121A–124A. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources