Protection against genital herpes infection in mice immunized under different hormonal conditions correlates with induction of vagina-associated lymphoid tissue - PubMed (original) (raw)
Protection against genital herpes infection in mice immunized under different hormonal conditions correlates with induction of vagina-associated lymphoid tissue
Amy E Gillgrass et al. J Virol. 2005 Mar.
Abstract
The present study was undertaken to examine the effect of the hormonal environment on immunization with an attenuated strain of herpes simplex virus type 2 (HSV-2 TK(-)) and subsequent protection against challenge. Ovariectomized mice were administered saline (S; control), estradiol (E(2)), progesterone (P(4)), or a combination of estradiol and progesterone (E+P) and immunized intravaginally (IVAG) with HSV-2 TK(-). Three weeks later, the immunized mice were challenged IVAG with wild-type HSV-2. Mice that were immunized following E treatment were not protected, whereas complete protection against the challenge was seen in mice from the S- and P(4)-treated groups. In the P(4)-treated group, 15% of mice developed chronic pathology following TK(-) immunization. Interestingly, about 40% of the E+P-treated mice were also protected. Upon examination of viral shedding in the vaginal secretions, it was clear that protection against challenge was dependent on the ability of the TK(-) virus to cause productive genital infection under different hormonal conditions. In the protected mice (the S and P groups and part of the E+P group), induced vagina-associated lymphoid tissues composed of CD11c(+) dendritic cells and CD3(+) and CD4(+) T cells were formed transiently in the vaginal lamina propria from day 2 to day 5 postchallenge. These aggregates were absent in the unprotected mice (the E group and part of the E+P group). Significant HSV-2-specific activation of lymphocytes was observed in the local draining lymph nodes of protected mice. This response was absent in the unprotected groups. High titers of gB-specific local immunoglobulin A (IgA) antibodies were present in the vaginal secretions of S- and P(4)-treated immunized mice following HSV-2 challenge. The S-treated group of mice also had high gB-specific IgG titers. These studies show that sex hormones modify the induction of protective immune responses following IVAG immunization.
Figures
FIG. 1.
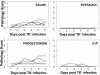
Pathology of ovariectomized, hormone-treated mice after immunization with HSV-2 TK− (105 PFU). Mice were ovariectomized and given different combinations of hormones, as described in Materials and Methods. Following IVAG immunization with attenuated HSV-2 (TK−), vaginal pathology was monitored daily. Pathology scores of each mouse in all four hormone groups over 6 days following immunization are shown. Pathology scores halfway between two numbers were given when a readout was transitional between two consecutive scores. Each group had six to eight mice. The results shown are representative of three separate experiments.
FIG. 2.
Virus titers in vaginal washes of ovariectomized, hormone-treated mice after immunization with HSV-2 TK− (105 PFU). Mice were ovariectomized and given different combinations of hormones, as described in Materials and Methods. Following IVAG immunization with attenuated HSV-2 (TK−), vaginal washes were collected daily and viral plaque assays were done as described in the text. Plaques were counted, and viral titers were expressed in PFU per milliliter. Each symbol represents a single animal (n = six to eight animals in each group). Dashed lines represent the lower detection limit of the assay. The results are representative of three separate experiments.
FIG. 3.
Pathology and survival of ovariectomized, hormone-treated, immunized mice following challenge with wild-type HSV-2. Mice were ovariectomized and given different combinations of hormones, as described in Materials and Methods. Three weeks after IVAG immunization with attenuated HSV-2, mice were challenged IVAG with wild-type HSV-2 (105 PFU). Vaginal pathology and survival were monitored daily. Pathology scores of each mouse in all four hormone groups over 5 days following challenge are shown. Pathology scores halfway between two numbers were given when a readout was transitional between two consecutive scores. Final survival numbers are indicated for each hormone treatment group. Each group had six to eight mice. The results shown are representative of three separate experiments.
FIG. 4.
Virus titers in vaginal washes of ovariectomized, hormone-treated, immunized mice after challenge with wild-type HSV-2. Mice were ovariectomized and given different combinations of hormones, as described in Materials and Methods. Three weeks after IVAG immunization with attenuated HSV-2, mice were challenged IVAG with wild-type HSV-2 (105 PFU). Vaginal washes were collected daily, and viral plaque assays were done as described in the text. Plaques were counted, and viral titers were expressed in PFU per milliliter. Each symbol represents a single animal (n = six to eight animals in each group). The dashed lines represent the lower detection limit of the assay. The results are representative of three separate experiments.
FIG. 5.
Histopathology and localization of infection in the vaginal tissue of ovariectomized, hormone-treated, immunized mice after wild-type HSV-2 challenge. Mice were ovariectomized and given different combinations of hormones, as described in Materials and Methods. Three weeks after IVAG immunization with attenuated HSV-2, mice were challenged IVAG with wild-type HSV-2 (105 PFU). Mice were sacrificed 3 days postchallenge, histopathology was examined (A to D), and HSV-2 infection was localized by immunohistochemistry (E to H). Representative tissue sections are shown. Only the vaginal sections from a protected E+P mouse are shown here (D and H). Note the intact vaginal epithelium and lack of any inflammation (D) as well as the absence of any infection (H). The E2-treated mice show acute inflammation and leukocytic infiltration (B) and extensive infection, shown in pink (F). Progesterone-treated mice show extensive epithelial damage, leukocytic infiltration in the tissue and in the lumen (C), and the absence of any HSV-2 staining (G). The saline control mice show some epithelial damage and infiltration (A) but no infection (E). Isotype controls did not show any positive staining (data not shown). Original magnification, ×100.
FIG. 6.
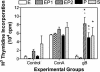
Local draining lymph node proliferation in hormone-treated, immunized mice following challenge with wild-type HSV-2. Iliac lymph nodes draining the genital tract were removed 3 days after wild-type HSV-2 challenge (105 PFU), and cells were isolated and cultured for 48 h, as described in Materials and Methods. T-cell proliferation was measured in response to mitogen (ConA) and HSV-2 antigen (glycoprotein gB). The E+P group was divided into protected and nonprotected groups. Control cultures did not receive any stimulation. Results are shown as means ± standard errors of the mean (n = six to eight animals in the S, E2, and P4 groups). Data shown are representative of two separate experiments. EP1 (n = 3), unprotected E+P-treated animals; EP2 (n = 4), protected E+P-treated animals; ★, P < 0.05 compared to nonprotected groups (E2 and not protected E+P); o, P < 0.05 compared to respective control unstimulated cultures.
FIG. 7.
iVALT in vaginal mucosae of hormone-treated, immunized mice challenged with wild-type HSV-2. Mice were ovariectomized and given different combinations of hormones, as described in Materials and Methods. Three weeks after IVAG immunization with attenuated HSV-2, mice were challenged IVAG with wild-type HSV-2 (105 PFU). Mice were sacrificed 2 to 5 days postchallenge, and immunohistochemical staining was done to localize immune cells. Representative tissue sections are shown from day 2 postchallenge. Note the large size of the iVALT in the E+P vagina compared to the size in saline- and progesterone-treated mice. The CD11c staining was seen on the periphery of the iVALTs, while CD4+ and CD3+ cells were localized inside as well as in close association with CD11c+ cells. CD8+ cells were distributed throughout the laminae propriae. Original magnification, ×100.
FIG. 8.
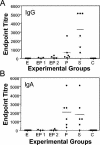
HSV-2-specific antibody titers in vaginal washes of hormone-treated, immunized mice following challenge with wild-type HSV-2. Hormone-treated, immunized mice were challenged with wild-type HSV-2 (105 PFU). Vaginal washes were collected daily for 5 days postchallenge and pooled for each animal. HSV-2 gB-specific IgA (B) and IgG (A) antibodies were measured by ELISA, and end point titers were determined as described in Materials and Methods. E, estradiol treated; EP1, E+P treated and unprotected; EP2, E+P treated and protected; P, progesterone treated; S, saline controls; C, normal nonovariectomized mice. Each dot indicates an individual mouse. Bars show mean values for the group.
Similar articles
- Intranasal and subcutaneous immunization under the effect of estradiol leads to better protection against genital HSV-2 challenge compared to progesterone.
Bhavanam S, Snider DP, Kaushic C. Bhavanam S, et al. Vaccine. 2008 Nov 11;26(48):6165-72. doi: 10.1016/j.vaccine.2008.08.045. Epub 2008 Sep 18. Vaccine. 2008. PMID: 18804503 - Estradiol limits viral replication following intravaginal immunization leading to diminished mucosal IgG response and non-sterile protection against genital herpes challenge.
Gillgrass A, Chege D, Bhavanam S, Kaushic C. Gillgrass A, et al. Am J Reprod Immunol. 2010 Apr 1;63(4):299-309. doi: 10.1111/j.1600-0897.2009.00796.x. Epub 2010 Jan 11. Am J Reprod Immunol. 2010. PMID: 20070285 - Protective immunity against HSV-2 in the mouse vagina.
Parr MB, Parr EL. Parr MB, et al. J Reprod Immunol. 1997 Nov 30;36(1-2):77-92. doi: 10.1016/s0165-0378(97)00055-7. J Reprod Immunol. 1997. PMID: 9430740 Review. - Vaginal immunity in the HSV-2 mouse model.
Parr MB, Parr EL. Parr MB, et al. Int Rev Immunol. 2003 Jan-Feb;22(1):43-63. doi: 10.1080/08830180305228. Int Rev Immunol. 2003. PMID: 12710503 Review.
Cited by
- Repeated Intravaginal Inoculation of Zika Virus Protects Cynomolgus Monkeys from Subcutaneous Superchallenge.
Shofa M, Okamura T, Urano E, Matsuura Y, Yasutomi Y, Saito A. Shofa M, et al. Int J Mol Sci. 2022 Nov 13;23(22):14002. doi: 10.3390/ijms232214002. Int J Mol Sci. 2022. PMID: 36430481 Free PMC article. - Immune receptor toll-like receptor 4 contributes to stress-induced affective responses in a sex-specific manner.
Quave CB, Nieto SJ, Haile CN, Kosten TA. Quave CB, et al. Brain Behav Immun Health. 2021 Mar 31;14:100248. doi: 10.1016/j.bbih.2021.100248. eCollection 2021 Jul. Brain Behav Immun Health. 2021. PMID: 34589759 Free PMC article. - Vaginal delivery of vaccines.
VanBenschoten HM, Woodrow KA. VanBenschoten HM, et al. Adv Drug Deliv Rev. 2021 Nov;178:113956. doi: 10.1016/j.addr.2021.113956. Epub 2021 Sep 1. Adv Drug Deliv Rev. 2021. PMID: 34481031 Free PMC article. Review. - Sex and Age Effects on Neurobehavioral Toxicity Induced by Binge Alcohol.
Cortez I, Rodgers SP, Kosten TA, Leasure JL. Cortez I, et al. Brain Plast. 2020 Dec 29;6(1):5-25. doi: 10.3233/BPL-190094. Brain Plast. 2020. PMID: 33680843 Free PMC article. Review. - Estradiol Enhances Antiviral CD4+ Tissue-Resident Memory T Cell Responses following Mucosal Herpes Simplex Virus 2 Vaccination through an IL-17-Mediated Pathway.
Bagri P, Ghasemi R, McGrath JJC, Thayaparan D, Yu E, Brooks AG, Stämpfli MR, Kaushic C. Bagri P, et al. J Virol. 2020 Dec 9;95(1):e01206-20. doi: 10.1128/JVI.01206-20. Print 2020 Dec 9. J Virol. 2020. PMID: 33028712 Free PMC article.
References
- Abel, K., T. Rourke, D. Lu, K. Bost, M. B. McChesney, and C. J. Miller. 2004. Abrogation of attenuated lentivirus-induced protection in rhesus macaques by administration of Depo-Provera before intravaginal challenge with simian immunodeficiency virus mac239. J. Infect. Dis. 190:1697-1705. - PMC - PubMed
- Aral, S. O., and K. K. Holmes. 1999. Social and behavioral determinants of the epidemiology of STDs: industrialized and developing countries, p. 95-106. In K. K. Holmes, P. F. Sparling, P. A. Mardh, S. M. Lemon, et al. (ed.), Sexually transmitted diseases, 3rd ed. McGraw-Hill, New York, N.Y. - PubMed
- Beagley, K. W., and C. M. Gockel. 2003. Regulation of innate and adaptive immunity by female sex hormones oestradiol and progesterone. FEMS Immunol. Med. Microbiol. 38:13-22. - PubMed
- Benki, S., S. B. Mostad, B. A. Richardson, K. Mandaliya, J. K. Kreiss, and J. Overbaugh. 2004. Cyclic shedding of HIV-1 RNA in cervical secretions during the menstrual cycle. J. Infect. Dis. 189:2192-2201. - PubMed
- Crowley, T., P. Horner, A. Hughes, J. Berry, I. Paul, and O. Caul. 1997. Hormonal factors and laboratory detection of Chlamydia trachomatis in women: implications for screening? Int. J. STD AIDS 8:25-31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous