Severe acute respiratory syndrome coronavirus 3a protein is a viral structural protein - PubMed (original) (raw)
Severe acute respiratory syndrome coronavirus 3a protein is a viral structural protein
Naoto Ito et al. J Virol. 2005 Mar.
Abstract
The present study showed the association of a severe acute respiratory syndrome coronavirus (SCoV) accessory protein, 3a, with plasma membrane and intracellular SCoV particles in infected cells. 3a protein appeared to undergo posttranslational modifications in infected cells and was incorporated into SCoV particles, establishing that 3a protein was a SCoV structural protein.
Figures
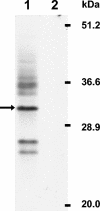
FIG. 1.
Western blot analysis of 3a protein. Human colonic adenocarcinoma Caco2 cells were infected with SCoV at a multiplicity of infection of 0.01, and cells were solubilized with sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer (100 mM Tris-HCl [pH 6.8], 4% sodium dodecyl sulfate, 0.2% bromophenol blue, 20% glycerol, and 200 mM beta-mercaptoethanol) at 5 days p.i. Cell extracts were applied to a sodium dodecyl sulfate-15% polyacrylamide gel, and Western blot analysis was performed using anti-3a antibody. Lane 1, SCoV-infected Caco2 cells; lane 2, mock-infected Caco2 cells. Arrow; major 31-kDa 3a protein.
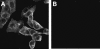
FIG. 2.
Confocal microscopic analysis of 3a protein in SCoV-infected cells. Vero E6 cells growing in eight-well chamber slides (Lab-Tek, Naperville, Ill.) were infected with SCoV at a multiplicity of infection of 1 (A) or mock infected (B). At 24 h p.i., cultures were incubated overnight with 4% paraformaldehyde and then treated with 0.25% Triton X-100 for 15 min. Subsequently, cells were incubated with anti-3a antibody and goat anti-rabbit secondary antibody conjugated with Alexa Fluor 488 dye (Molecular Probes, Eugene, Oreg.). Cells were observed under the Zeiss LSM 510 UV META laser scanning confocal microscope in the University of Texas Medical Branch Infectious Disease and Toxicology Optical Imaging Core.
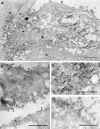
FIG. 3.
Immunogold labeling of 3a protein in SCoV-infected cells. Caco2 cells were infected with SCoV at a multiplicity of infection of 0.5, fixed at 48 h p.i., and embedded in LR White resin. Ultrathin sections of the cells were incubated with anti-3a antibody and goat anti-rabbit immunoglobulin G (heavy plus light) conjugated to 15-nm colloidal gold particles (Amersham Biosciences). (A) In a SCoV-infected cell the label is clearly associated with intracellular virus (v) and with the virions at the cell surface (arrows). An uninfected cell in the upper left corner is devoid of label. n, nucleus. Bar = 1 μm. (B) 3a protein is associated with plasma membranes. V, SCoV particles associated with 3a protein. (C) Association of 3a protein with intracellular virus particles (arrows). (D) Portion of cytoplasm of a mock-infected Caco2 cell showing occasional staining of a few gold particles near the plasma membrane. Bars (B to D) = 0.5 μm.
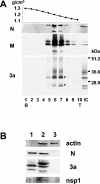
FIG. 4.
Western blot analysis of N, M, and 3a proteins in purified SCoV. Caco2 cells were infected with SCoV at a multiplicity of infection of 1, and culture fluid was collected at 5 days p.i. Released SCoV was purified by sucrose gradient centrifugation as described in the text. (A) Ten fractions from a 20 to 60% sucrose gradient containing the virus particles were collected and numbered from bottom (B) to top (T) of the gradient. The top panel represents the density of each sucrose fraction. IC, intracellular proteins from SCoV-infected Caco2 cells. (B) Purified SCoV (lane 1), cell extracts from SCoV-infected Caco2 cells at 5 days p.i. (lane 2), and uninfected Caco2 cells at 5 days p.i. (lane 3) were analyzed for actin protein, N protein, 3a protein, and SCoV nsp1 protein.
Similar articles
- Severe acute respiratory syndrome coronavirus 3a protein is released in membranous structures from 3a protein-expressing cells and infected cells.
Huang C, Narayanan K, Ito N, Peters CJ, Makino S. Huang C, et al. J Virol. 2006 Jan;80(1):210-7. doi: 10.1128/JVI.80.1.210-217.2006. J Virol. 2006. PMID: 16352545 Free PMC article. - Severe acute respiratory syndrome coronavirus 7a accessory protein is a viral structural protein.
Huang C, Ito N, Tseng CT, Makino S. Huang C, et al. J Virol. 2006 Aug;80(15):7287-94. doi: 10.1128/JVI.00414-06. J Virol. 2006. PMID: 16840309 Free PMC article. - Role of Severe Acute Respiratory Syndrome Coronavirus Viroporins E, 3a, and 8a in Replication and Pathogenesis.
Castaño-Rodriguez C, Honrubia JM, Gutiérrez-Álvarez J, DeDiego ML, Nieto-Torres JL, Jimenez-Guardeño JM, Regla-Nava JA, Fernandez-Delgado R, Verdia-Báguena C, Queralt-Martín M, Kochan G, Perlman S, Aguilella VM, Sola I, Enjuanes L. Castaño-Rodriguez C, et al. mBio. 2018 May 22;9(3):e02325-17. doi: 10.1128/mBio.02325-17. mBio. 2018. PMID: 29789363 Free PMC article. - Expression of the severe acute respiratory syndrome coronavirus 3a protein and the assembly of coronavirus-like particles in the baculovirus expression system.
Khan S, Ng ML, Tan YJ. Khan S, et al. Methods Mol Biol. 2007;379:35-50. doi: 10.1007/978-1-59745-393-6_3. Methods Mol Biol. 2007. PMID: 17502669 Free PMC article. Review. - Understanding the accessory viral proteins unique to the severe acute respiratory syndrome (SARS) coronavirus.
Tan YJ, Lim SG, Hong W. Tan YJ, et al. Antiviral Res. 2006 Nov;72(2):78-88. doi: 10.1016/j.antiviral.2006.05.010. Epub 2006 Jun 6. Antiviral Res. 2006. PMID: 16820226 Free PMC article. Review.
Cited by
- Mouse hepatitis virus JHMV I protein is required for maximal virulence.
Lowery SA, Schuster N, Wong L-YR, Carrillo T Jr, Peters E, Odle A, Sariol A, Cesarz I, Li P, Perlman S. Lowery SA, et al. J Virol. 2024 Sep 17;98(9):e0068024. doi: 10.1128/jvi.00680-24. Epub 2024 Aug 19. J Virol. 2024. PMID: 39158347 - Characterization of humoral immune responses against SARS-CoV-2 accessory proteins in infected patients and mouse model.
Li Y, Tang Y, Wang X, Zhu A, Liu D, He Y, Guo H, Zheng J, Liu X, Chi F, Wang Y, Zhuang Z, Zhang Z, Liu D, Chen Z, Li F, Ran W, Yu K, Wang D, Wen L, Zhuo J, Zhang Y, Xi Y, Zhao J, Zhao J, Sun J. Li Y, et al. Virol Sin. 2024 Jun;39(3):414-421. doi: 10.1016/j.virs.2024.04.005. Epub 2024 Apr 26. Virol Sin. 2024. PMID: 38677713 Free PMC article. - Coronavirus accessory protein ORF3 biology and its contribution to viral behavior and pathogenesis.
Si F, Song S, Yu R, Li Z, Wei W, Wu C. Si F, et al. iScience. 2023 Apr 21;26(4):106280. doi: 10.1016/j.isci.2023.106280. Epub 2023 Feb 28. iScience. 2023. PMID: 36945252 Free PMC article. Review. - The SARS-CoV-2 accessory protein Orf3a is not an ion channel, but does interact with trafficking proteins.
Miller AN, Houlihan PR, Matamala E, Cabezas-Bratesco D, Lee GY, Cristofori-Armstrong B, Dilan TL, Sanchez-Martinez S, Matthies D, Yan R, Yu Z, Ren D, Brauchi SE, Clapham DE. Miller AN, et al. Elife. 2023 Jan 25;12:e84477. doi: 10.7554/eLife.84477. Elife. 2023. PMID: 36695574 Free PMC article. - SARS-CoV-2 accessory protein 7b forms homotetramers in detergent.
Surya W, Queralt-Martin M, Mu Y, Aguilella VM, Torres J. Surya W, et al. Virol J. 2022 Nov 21;19(1):193. doi: 10.1186/s12985-022-01920-0. Virol J. 2022. PMID: 36414943 Free PMC article.
References
- Drosten, C., S. Gunther, W. Preiser, S. van der Werf, H. R. Brodt, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova, R. A. Fouchier, A. Berger, A. M. Burguiere, J. Cinatl, M. Eickmann, N. Escriou, K. Grywna, S. Kramme, J. C. Manuguerra, S. Muller, V. Rickerts, M. Sturmer, S. Vieth, H. D. Klenk, A. D. Osterhaus, H. Schmitz, and H. W. Doerr. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348:1967-1976. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007536/AI/NIAID NIH HHS/United States
- N01AI25489/AI/NIAID NIH HHS/United States
- AI007536/AI/NIAID NIH HHS/United States
- AI29984/AI/NIAID NIH HHS/United States
- R21 AI029984/AI/NIAID NIH HHS/United States
- R01 AI029984/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases