Directional persistence of EGF-induced cell migration is associated with stabilization of lamellipodial protrusions - PubMed (original) (raw)
Directional persistence of EGF-induced cell migration is associated with stabilization of lamellipodial protrusions
Brian D Harms et al. Biophys J. 2005 Feb.
Abstract
Migrating cells can sustain a relatively constant direction of lamellipodial protrusion and locomotion over timescales ranging from minutes to hours. However, individual waves of lamellipodial extension occur over much shorter characteristic times. Little understanding exists regarding how cells might integrate biophysical processes across these disparate timescales to control the directional persistence of locomotion. We address this issue by examining the effects of epidermal growth factor (EGF) stimulation on long-timescale directional persistence and short-timescale lamellipodial dynamics of EGF receptor-transfected Chinese hamster ovary cells migrating on fibronectin-coated substrata. Addition of EGF increased persistence, with the magnitude of increase correlating with fibronectin coating concentration. Kymographic analysis of EGF-stimulated lamellipodial dynamics revealed that the temporal stability of lamellipodial protrusions similarly increased with fibronectin concentration. A soluble RGD peptide competitor reduced both the persistence of long-timescale cell paths and the stability of short-timescale membrane protrusions, indicating that cell-substratum adhesion concomitantly influences lamellipodial dynamics and directional persistence. These results reveal the importance of adhesion strength in regulating the directional motility of cells and suggest that the short-timescale kinetics of adhesion complex formation may play a key role in modulating directional persistence over much longer timescales.
Figures
FIGURE 1
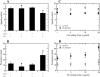
Migration behavior of EGFR-transfected CHO cells is affected by Fn and EGF. Time-lapse videomicroscopy was used to capture the motility responses of individual CHO K1 and CHO-EGFR cells. (A and B) Without EGFR-GFP transfection, EGF does not affect the migratory response of CHO K1 cells; (C_–_F) Transfected CHO-EGFR cells respond to EGF by upregulating their migration in a Fn-dependent manner. Digital images were taken every 15 min for a total of 6 h per experiment. Each wind-rose plot shows centroid tracks from 10 representative cells in a typical experiment, with the initial position of each track superimposed at 0,0 for clarity. Distance between hatch marks on both axes is 50 _μ_m.
FIGURE 2
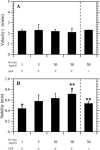
Effects of EGF and Fn on speed and persistence for migrating CHO-EGFR cells. Transfection of EGFR-GFP into CHO K1 cells alters cell speed (A) and persistence (B) in the presence, but not the absence, of EGF. Data in A and B were obtained using a 10 _μ_g/ml Fn coating concentration. Asterisks denote inequality at 95% confidence for key comparison. (C) EGF stimulation, but not Fn coating concentration, modulates CHO-EGFR speed. Single-factor ANOVA: p = 0.54 (S, −EGF), p = 0.90 (S, +EGF). (D) Persistence time increases with Fn coating concentration in the presence of EGF. ANOVA: p = 0.88 (P, −EGF), p = 0.03 (P, +EGF). To derive the speed of a single cell, total cell path length was divided by the total time of observation. Single cell persistence times were derived from nonlinear least-squares regression using individual cell speed and root mean-square displacement data as inputs. Reported S and P data represent the mean ± 2 SE for three experiments per condition, with 80–100 cells per experiment.
FIGURE 3
Lamellipodial membrane dynamics of CHO-EGFR cells. Representative short-timescale kymographs from time-lapse movies of CHO-EGFR cells stimulated with EGF and migrating on 1 _μ_g/ml (A) and 30 _μ_g/ml (B) Fn. Kymographs depict variations in lamellipodial activity along a one-pixel-wide line drawn perpendicularly to the cell membrane in sequential phase contrast images. Ascending contours show lamellipodial protrusion, whereas descending contours show lamellipodial retraction. Dark regions indicate membrane ruffling.
FIGURE 4
Lamellipodial stability correlates with directional persistence in CHO-EGFR migration. (A) Fn coating concentration does not affect the average velocity of membrane protrusion. The average slope of ascending kymograph membrane waves defines protrusion velocity. ANOVA: p = 0.86. (B) Protrusion stability increases with Fn concentration in the presence of EGF. Stability represents the time elapsed between the beginning of a single protrusion wave and its spatial peak. Nonoverlapping 95% confidence intervals are indicated by asterisks. ANOVA: p = 0.03.
FIGURE 5
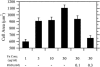
Soluble RGD peptide inhibits cell adhesion. The average area of CHO-EGFR cells migrating in the presence of EGF was determined for all Fn coating concentrations; cell adhesion as described by cell area increased in parallel with Fn. Addition of peptide inhibitor abrogated this Fn-dependent effect on adhesion in a dose-dependent fashion.
FIGURE 6
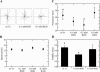
RGD peptide reduces both long-timescale directional persistence and short-timescale protrusion stability. (A) Wind-rose plots for migration experiments of CHO-EGFR cells plated on 30 _μ_g/ml Fn in the presence of EGF and varying amounts of GRGDSP, a soluble competitive inhibitor of Fn binding to _α_5_β_1 integrins. Distance between hatch marks on both axes is 50 _μ_m. (B) Cell speeds and (C) persistence times for the RGD migration experiments. Speed and persistence were derived as in Fig. 2. ANOVA: p = 0.71 for RGD-dependent cell speed, p = 0.002 for RGD-dependent persistence. Nonoverlapping 95% confidence intervals are indicated by asterisks. (D) Kymography was performed on CHO-EGFR cells plated on 30 _μ_g/ml Fn in the presence of EGF and either GRGDSP or control GRADSP peptide. Nonoverlapping 95% confidence intervals are indicated by asterisks (p = 0.01).
Similar articles
- Epidermal growth factor alters fibroblast migration speed and directional persistence reciprocally and in a matrix-dependent manner.
Ware MF, Wells A, Lauffenburger DA. Ware MF, et al. J Cell Sci. 1998 Aug;111 ( Pt 16):2423-32. doi: 10.1242/jcs.111.16.2423. J Cell Sci. 1998. PMID: 9683636 - Epidermal growth factor increased the expression of alpha2beta1-integrin and modulated integrin-mediated signaling in human cervical adenocarcinoma cells.
Yamanaka I, Koizumi M, Baba T, Yamashita S, Suzuki T, Kudo R. Yamanaka I, et al. Exp Cell Res. 2003 Jun 10;286(2):165-74. doi: 10.1016/s0014-4827(03)00065-x. Exp Cell Res. 2003. PMID: 12749846 - Biophysical integration of effects of epidermal growth factor and fibronectin on fibroblast migration.
Maheshwari G, Wells A, Griffith LG, Lauffenburger DA. Maheshwari G, et al. Biophys J. 1999 May;76(5):2814-23. doi: 10.1016/S0006-3495(99)77435-7. Biophys J. 1999. PMID: 10233097 Free PMC article. - A novel function of WAVE in lamellipodia: WAVE1 is required for stabilization of lamellipodial protrusions during cell spreading.
Yamazaki D, Fujiwara T, Suetsugu S, Takenawa T. Yamazaki D, et al. Genes Cells. 2005 May;10(5):381-92. doi: 10.1111/j.1365-2443.2005.00845.x. Genes Cells. 2005. PMID: 15836768 - Steering cell migration: lamellipodium dynamics and the regulation of directional persistence.
Krause M, Gautreau A. Krause M, et al. Nat Rev Mol Cell Biol. 2014 Sep;15(9):577-90. doi: 10.1038/nrm3861. Nat Rev Mol Cell Biol. 2014. PMID: 25145849 Review.
Cited by
- A perspective of fluorescence microscopy for cellular structural biology with EGFR as witness.
Martin-Fernandez ML. Martin-Fernandez ML. J Microsc. 2023 Jul;291(1):73-91. doi: 10.1111/jmi.13151. Epub 2022 Nov 4. J Microsc. 2023. PMID: 36282005 Free PMC article. - Kinetic model for lamellipodal actin-integrin 'clutch' dynamics.
Macdonald A, Horwitz AR, Lauffenburger DA. Macdonald A, et al. Cell Adh Migr. 2008 Apr-May;2(2):95-105. doi: 10.4161/cam.2.2.6210. Epub 2008 Apr 29. Cell Adh Migr. 2008. PMID: 19262096 Free PMC article. - Proteolysis of cortactin by calpain regulates membrane protrusion during cell migration.
Perrin BJ, Amann KJ, Huttenlocher A. Perrin BJ, et al. Mol Biol Cell. 2006 Jan;17(1):239-50. doi: 10.1091/mbc.e05-06-0488. Epub 2005 Nov 9. Mol Biol Cell. 2006. PMID: 16280362 Free PMC article. - Weak power frequency magnetic fields induce microtubule cytoskeleton reorganization depending on the epidermal growth factor receptor and the calcium related signaling.
Wu X, Du J, Song W, Cao M, Chen S, Xia R. Wu X, et al. PLoS One. 2018 Oct 12;13(10):e0205569. doi: 10.1371/journal.pone.0205569. eCollection 2018. PLoS One. 2018. PMID: 30312357 Free PMC article. - The development of a novel high throughput computational tool for studying individual and collective cellular migration.
Chapnick DA, Jacobsen J, Liu X. Chapnick DA, et al. PLoS One. 2013 Dec 27;8(12):e82444. doi: 10.1371/journal.pone.0082444. eCollection 2013. PLoS One. 2013. PMID: 24386097 Free PMC article.
References
- Bailly, M., J. S. Condeelis, and J. E. Segall. 1998a. Chemoattractant-induced lamellipod extension. Microsc. Res. Tech. 43:433–443. - PubMed
- Bailly, M., L. Yan, G. M. Whitesides, J. S. Condeelis, and J. E. Segall. 1998b. Regulation of protrusion shape and adhesion to the substratum during chemotactic responses of mammalian carcinoma cells. Exp. Cell Res. 241:285–299. - PubMed
- Bear, J. E., T. M. Svitkina, M. Krause, D. A. Schafer, J. J. Loureiro, G. A. Strasser, I. V. Maly, O. Y. Chaga, J. A. Cooper, G. G. Borisy, and F. B. Gertler. 2002. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 109:509–521. - PubMed
- Brahmbhatt, A. A., and R. L. Klemke. 2003. ERK and RhoA differentially regulate pseudopodia growth and retraction during chemotaxis. J. Biol. Chem. 278:13016–13025. - PubMed
- Burridge, K., and M. Chrzanowski-Wodnicka. 1996. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12:463–519. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources