Neuroprotection and acute spinal cord injury: a reappraisal - PubMed (original) (raw)
Review
Neuroprotection and acute spinal cord injury: a reappraisal
Edward D Hall et al. NeuroRx. 2004 Jan.
Abstract
It has long been recognized that much of the post-traumatic degeneration of the spinal cord following injury is caused by a multi-factorial secondary injury process that occurs during the first minutes, hours, and days after spinal cord injury (SCI). A key biochemical event in that process is reactive oxygen-induced lipid peroxidation (LP). In 1990 the results of the Second National Acute Spinal Cord Injury Study (NASCIS II) were published, which showed that the administration of a high-dose regimen of the glucocorticoid steroid methylprednisolone (MP), which had been previously shown to inhibit post-traumatic LP in animal models of SCI, could improve neurological recovery in spinal-cord-injured humans. This resulted in the registration of high-dose MP for acute SCI in several countries, although not in the U.S. Nevertheless, this treatment quickly became the standard of care for acute SCI since the drug was already on the U.S. market for many other indications. Subsequently, it was demonstrated that the non-glucocorticoid 21-aminosteroid tirilazad could duplicate the antioxidant neuroprotective efficacy of MP in SCI models, and evidence of human efficacy was obtained in a third NASCIS trial (NASCIS III). In recent years, the use of high-dose MP in acute SCI has become controversial largely on the basis of the risk of serious adverse effects versus what is perceived to be on average a modest neurological benefit. The opiate receptor antagonist naloxone was also tested in NASCIS II based upon the demonstration of its beneficial effects in SCI models. Although it did not a significant overall effect, some evidence of efficacy was seen in incomplete (i.e., paretic) patients. The monosialoganglioside GM1 has also been examined in a recently completed clinical trial in which the patients first received high-dose MP treatment. However, GM1 failed to show any evidence of a significant enhancement in the extent of neurological recovery over the level afforded by MP therapy alone. The present paper reviews the past development of MP, naloxone, tirilazad, and GM1 for acute SCI, the ongoing MP-SCI controversy, identifies the regulatory complications involved in future SCI drug development, and suggests some promising neuroprotective approaches that could either replace or be used in combination with high-dose MP.
Figures
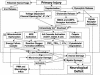
FIG. 1.
Pathophysiology of secondary injury in the injured spinal cord. 5-LO = 5-lipoxygenase; TXA2 = thromboxane A2; LTs = leukotrienes; ONOO− = peroxynitrite anion; PMN = polymorphonuclear leukocyte.
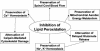
FIG. 2.
Hypothesized central role of inhibition of LP in the neuroprotective effects of high-dose MP in acute SCI.
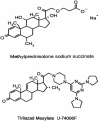
FIG. 3.
Chemical structures of the glucocorticoid steroid MP shown as the sodium salt of the 21-hemisuccinate ester and the non-glucocorticoid 21-aminosteroid tirilazad mesylate.
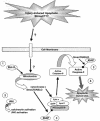
FIG. 4.
Schematic diagram depicting the potential steps leading to caspase-3 activation in CNS trauma. Several injury-induced extracellular apoptotic stimuli most likely exist and the mechanisms by which these extracellular signals are transduced to the intracellular pathway are only beginning to emerge. However, there are several lines of evidence supporting the release of pro-apoptotic molecules from the mitochondria as important steps in activation of the caspase-3 apoptotic. Other pro-apoptotic factors that are released by the mitochondria, but do not involve caspase-3 activation include endonuclease G and AIF (not shown). Given our current understanding of the intracellular signaling events, there are a number of points in the apoptotic cascade that can be targeted. For example, the overexpression of anti-apoptotic molecules including Bcl-XL,1 Akt,2 and XIAP3 to the injured spinal cord may limit ongoing apoptosis by reducing cytochrome c release (Bcl-XL and Akt), the activation of calcineurin and the JNK stress-induced pathway (Akt), or caspase activation via Smac/DIABLO (XIAP). In addition, the identification of small, cell-permeable synthetic molecules that inhibit the actions or activation of caspase-34 may prove advantageous over existing tri- and tetrapeptide-based competitive inhibitors. Such strategies will be critical for understanding the contribution of apoptotic cell death to the functional deficits observed in SCI, and hopefully allow for development of therapeutic approaches targeting this cell death process.
Similar articles
- Drug development in spinal cord injury: what is the FDA looking for?
Hall ED. Hall ED. J Rehabil Res Dev. 2003 Jul-Aug;40(4 Suppl 1):81-91. doi: 10.1682/jrrd.2003.08.0081. J Rehabil Res Dev. 2003. PMID: 15077652 - Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study.
Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings M, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL Jr, Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W. Bracken MB, et al. JAMA. 1997 May 28;277(20):1597-604. JAMA. 1997. PMID: 9168289 Clinical Trial. - Steroids for acute spinal cord injury.
Bracken MB. Bracken MB. Cochrane Database Syst Rev. 2012 Jan 18;1(1):CD001046. doi: 10.1002/14651858.CD001046.pub2. Cochrane Database Syst Rev. 2012. PMID: 22258943 Free PMC article. Review. - Antioxidant therapies for acute spinal cord injury.
Hall ED. Hall ED. Neurotherapeutics. 2011 Apr;8(2):152-67. doi: 10.1007/s13311-011-0026-4. Neurotherapeutics. 2011. PMID: 21424941 Free PMC article. Review.
Cited by
- Neuroprotective effects of sildenafil in experimental spinal cord injury in rabbits.
Kara H, Degirmenci S, Ak A, Bayir A, Kayis SA, Uyar M, Akinci M, Acar D, Kocacan M, Akyurek F. Kara H, et al. Bosn J Basic Med Sci. 2015 Jan 8;15(1):38-44. doi: 10.17305/bjbms.2015.1.119. Bosn J Basic Med Sci. 2015. PMID: 25725143 Free PMC article. - Neuroprotective effects of sulforaphane after contusive spinal cord injury.
Benedict AL, Mountney A, Hurtado A, Bryan KE, Schnaar RL, Dinkova-Kostova AT, Talalay P. Benedict AL, et al. J Neurotrauma. 2012 Nov 1;29(16):2576-86. doi: 10.1089/neu.2012.2474. Epub 2012 Sep 20. J Neurotrauma. 2012. PMID: 22853439 Free PMC article. - Comprehensive analysis of the differential expression profile of microRNAs in rats with spinal cord injury treated by electroacupuncture.
Zhou Z, Li H, Li H, Zhang J, Fu K, Cao C, Deng F, Luo J. Zhou Z, et al. Mol Med Rep. 2020 Aug;22(2):751-762. doi: 10.3892/mmr.2020.11161. Epub 2020 May 20. Mol Med Rep. 2020. PMID: 32468009 Free PMC article. - Pioglitazone treatment following spinal cord injury maintains acute mitochondrial integrity and increases chronic tissue sparing and functional recovery.
Patel SP, Cox DH, Gollihue JL, Bailey WM, Geldenhuys WJ, Gensel JC, Sullivan PG, Rabchevsky AG. Patel SP, et al. Exp Neurol. 2017 Jul;293:74-82. doi: 10.1016/j.expneurol.2017.03.021. Epub 2017 Mar 30. Exp Neurol. 2017. PMID: 28365473 Free PMC article. - Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury.
Kim Y, Jo SH, Kim WH, Kweon OK. Kim Y, et al. Stem Cell Res Ther. 2015 Nov 26;6:229. doi: 10.1186/s13287-015-0236-5. Stem Cell Res Ther. 2015. PMID: 26612085 Free PMC article.
References
- Anderson DK, Hall ED. Pathophysiology of spinal cord trauma. Ann Emerg Med 22: 987–992, 1993. - PubMed
- Hall ED. Mechanisms of secondary CNS injury. In: Neurosurgery 96: manual of neurosurgery (Palmer JD, ed), pp 505–510. New York: Churchill-Livingstone, 1996.
- Hall ED, Braughler JM. Central nervous system trauma and stroke. II. Physiological and pharmacological evidence for involvement of oxygen radicals and lipid peroxidation. Free Radic Biol Med 6: 303–313, 1989. - PubMed
- Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 75: 15–26, 1991. - PubMed
- Faden AI. Therapeutic approaches to spinal cord injury. Adv Neurol 72: 377–386, 1997. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous