Huntington's disease genetics - PubMed (original) (raw)
Review
Huntington's disease genetics
Richard H Myers. NeuroRx. 2004 Apr.
Abstract
Huntington's disease (HD) is a dominantly transmitted neurodegenerative disorder with wide variation in onset age but with an average age at onset of 40 years. Children of HD gene carriers have a 50% chance of inheriting the disease. The characteristic symptoms of HD are involuntary choreiform movements, cognitive impairment, mood disorders, and behavioral changes which are chronic and progressive over the course of the illness. HD is a "trinucleotide repeat" disorder, which is caused by an increase in the number of CAG repeats in the HD gene. Repeats of 40 or larger are associated with disease expression, whereas repeats of 26 and smaller are normal. Intermediate numbers of repeats, between 27 and 35, are not associated with disease expression but may expand in paternal transmission, resulting in the disease in descendents. Repeats of 36-39 are associated with reduced penetrance whereby some develop HD and others do not. The identification of the genetic defect in HD permits direct genetic testing for the presence of the gene alteration responsible for the disease. Tests may be performed in three circumstances: (1) confirmation of diagnosis, (2) predictive testing of persons at genetic risk for inheriting HD, and (3) prenatal testing. Testing is widely available and much experience has been gained with protocols that assist the individual in making an informed choice about test options, and minimize the occurrence of adverse emotional outcomes.
Figures
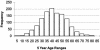
FIG. 1.
Huntington’s disease onset ages. The age at onset distribution in Huntington’s disease is very broad and may vary ffrom as young as 3 or 4 years of age to as old as 85. Onset presented here represents initial signs of motor impairment.
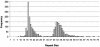
FIG. 2.
Normal and expanded HD repeat sizes. The distribution of repeats for Huntington’s disease may be divided into four categories. Repeats of 26 or fewer are normal. Repeats between 27 and 35 are rare and are not associated with the expression of the disease, but occasionally fathers with repeats in this range will transmit a repeat to descendants that is expanded to the range for expression of the illness. Repeats between 36 and 39 are associated with reduced penetrance whereby some individuals will develop HD and others will not. Repeats of 40 or larger are associated with the expression of HD. Persons carrying repeats in this range will develop HD, assuming they do not die of other causes before onset.
FIG. 3.
HD repeat size and onset age. The relationship between the repeat size and the age at onset is presented. Persons with repeats of 60 or larger commonly have very young onset, before the age of 20, and among these large repeats there is a clear relationship between repeat size and onset age. For persons with 55 repeats or fewer, the relationship between repeat size and onset age is much weaker and the repeat size is not predictive of onset age.
Similar articles
- A Study of Triplet-Primed PCR for Identification of CAG Repeat Expansion in the HTT Gene in a Cohort of 503 Indian Cases with Huntington's Disease Symptoms.
Chheda P, Chanekar M, Salunkhe Y, Dama T, Pais A, Pande S, Bendre R, Shah N. Chheda P, et al. Mol Diagn Ther. 2018 Jun;22(3):353-359. doi: 10.1007/s40291-018-0327-y. Mol Diagn Ther. 2018. PMID: 29619771 - Autopsy-proven Huntington's disease with 29 trinucleotide repeats.
Kenney C, Powell S, Jankovic J. Kenney C, et al. Mov Disord. 2007 Jan;22(1):127-30. doi: 10.1002/mds.21195. Mov Disord. 2007. PMID: 17115386 - Parent-of-origin differences of mutant HTT CAG repeat instability in Huntington's disease.
Aziz NA, van Belzen MJ, Coops ID, Belfroid RD, Roos RA. Aziz NA, et al. Eur J Med Genet. 2011 Jul-Aug;54(4):e413-8. doi: 10.1016/j.ejmg.2011.04.002. Epub 2011 Apr 23. Eur J Med Genet. 2011. PMID: 21540131 - [From gene to disease; HD gene and Huntington disease].
Maat-Kievit JA, Losekoot M, Roos RA. Maat-Kievit JA, et al. Ned Tijdschr Geneeskd. 2001 Nov 3;145(44):2120-3. Ned Tijdschr Geneeskd. 2001. PMID: 11723754 Review. Dutch. - Juvenile onset Huntington's disease--clinical and research perspectives.
Nance MA, Myers RH. Nance MA, et al. Ment Retard Dev Disabil Res Rev. 2001;7(3):153-7. doi: 10.1002/mrdd.1022. Ment Retard Dev Disabil Res Rev. 2001. PMID: 11553930 Review.
Cited by
- An update on the neurological short tandem repeat expansion disorders and the emergence of long-read sequencing diagnostics.
Chintalaphani SR, Pineda SS, Deveson IW, Kumar KR. Chintalaphani SR, et al. Acta Neuropathol Commun. 2021 May 25;9(1):98. doi: 10.1186/s40478-021-01201-x. Acta Neuropathol Commun. 2021. PMID: 34034831 Free PMC article. Review. - ROCK-phosphorylated vimentin modifies mutant huntingtin aggregation via sequestration of IRBIT.
Bauer PO, Hudec R, Goswami A, Kurosawa M, Matsumoto G, Mikoshiba K, Nukina N. Bauer PO, et al. Mol Neurodegener. 2012 Aug 28;7:43. doi: 10.1186/1750-1326-7-43. Mol Neurodegener. 2012. PMID: 22929228 Free PMC article. - Why tell asymptomatic children of the risk of an adult-onset disease in the family but not test them for it?
Malpas PJ. Malpas PJ. J Med Ethics. 2006 Nov;32(11):639-42. doi: 10.1136/jme.2005.015370. J Med Ethics. 2006. PMID: 17074821 Free PMC article. Review. - Antagonistic Pleiotropy in Human Disease.
Byars SG, Voskarides K. Byars SG, et al. J Mol Evol. 2020 Jan;88(1):12-25. doi: 10.1007/s00239-019-09923-2. Epub 2019 Dec 21. J Mol Evol. 2020. PMID: 31863128 Review. - MicroRNA editing patterns in Huntington's disease.
Guo S, Yang J, Jiang B, Zhou N, Ding H, Zhou G, Wu S, Suo A, Wu X, Xie W, Li W, Liu Y, Deng W, Zheng Y. Guo S, et al. Sci Rep. 2022 Feb 24;12(1):3173. doi: 10.1038/s41598-022-06970-6. Sci Rep. 2022. PMID: 35210471 Free PMC article.
References
- Myers RH, Marans K, MacDonald ME. Huntington’s disease. In: Genetic instabilities and hereditary neurological diseases (Warren ST, Wells RT, eds), pp 301–323. New York: Academic Press, 1998.
- Gusella JF, Wexler NS, Conneally PM, Naylor SL, Anderson MA, Tanzi RE et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature 306: 234–238, 1983. - PubMed
- The Huntington’s Disease Research Collaborative Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72: 971–983, 1993. - PubMed
- Duyao MP, Ambrose CM, Myers RH, Novelletto A, Persichetti F, Frontali M et al. Trinucleotide repeat length: instability and age of onset in Huntington’s disease. Nat Genet 4: 387–392, 1993. - PubMed
- Chong SS, Almqvist E, Telenius H, LaTray L, Nichol K, Bourdelat-Parks B et al. Contribution of DNA sequence and CAG size to mutation frequencies of intermediate alleles for Huntington disease: evidence from single sperm analyses. Hum Mol Genet 6: 301–309, 1997. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical