Anatomical basis of glial cell line-derived neurotrophic factor expression in the striatum and related basal ganglia during postnatal development of the rat - PubMed (original) (raw)
Anatomical basis of glial cell line-derived neurotrophic factor expression in the striatum and related basal ganglia during postnatal development of the rat
Tinmarla Frances Oo et al. J Comp Neurol. 2005.
Abstract
There is increasing evidence that glial cell line-derived neurotrophic factor (GDNF) plays a role as a limiting, striatal target-derived neurotrophic factor for dopamine neurons of the substantia nigra pars compacta (SNpc) by regulating the magnitude of the first phase of postnatal natural cell death which occurs in these neurons. While it has been shown that GDNF mRNA is relatively abundant in postnatal striatum, the cellular basis of its expression has been unknown. We therefore used nonradioactive in situ hybridization and immunohistochemistry to examine the cellular basis of GDNF mRNA and protein expression, respectively, in postnatal striatum and related structures. We found that GDNF mRNA is expressed within medium-sized striatal neurons. Expression in glia was not observed. At the protein level, regionally, GDNF expression in striatum was observed in striosomal patches, as previously described. At a cellular level a few neurons were observed, but they do not account for the striosomal pattern. This pattern is predominantly due to GDNF-positive neuropil. Some of this neuropil arises from tyrosine hydroxylase-positive nigro-striatal dopaminergic afferents. Astrocytic processes do not appear to contribute to the striosomal pattern. GDNF-positive fibers are identified not only within intrinsic striatal neuropil, but also in fibers within the major striatal efferent targets: the globus pallidus, the entopeduncular nucleus, and the SN pars reticulata. We conclude that during normal postnatal development, medium-sized neurons are the principal source of GDNF within the striatum.
Copyright 2005 Wiley-Liss, Inc.
Figures
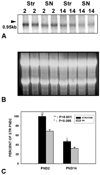
Fig. 1
Relative levels of GDNF mRNA in striatum and SN at the two phases of NCD in SN. A: Representative Northern analysis of GDNF mRNA expression in striatum and SN at PND2 (n = 2 for each region) and PND14 (n = 2 for each region). B: An ethidium bromide stain of the gel demonstrates equal loading of the lanes. C: Quantitative analysis of GDNF mRNA expression in striatum and SN at the two phases of NCD in SN (n = 8 for each condition). Note that mRNA levels were relatively greater in striatum than SN during both phases of NCD, but particularly at PND2 (P < 0.001 at PND2, P < 0.005 at PND14).
Fig. 2
Nonradioactive in situ hybridization staining of GDNF mRNA-positive profiles in striatum during development. A: A representative example of the cellular morphology defined by NRISH chromogen deposition is shown for a dopamine neuron of the SNpc by probing for the dopamine transporter (DAT). The cell soma is distinctly defined, and both primary and secondary dendrites (arrowheads) are demonstrated. B: A sense-GDNF probe reveals only faint background staining. C–F: Four examples of GDNF mRNA-positive neuronal profiles within the striatum at PND5. Scale bar = 10 µm.
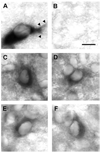
Fig. 3
Striatal GDNF immunoperoxidase staining during development. A: GDNF immunostaining at PND5 reveals distinctly stained patches (arrows) and a lateral crescent band of staining subjacent to the external capsule (arrowheads). B,C: Representative GDNF-positive neuronal profiles in striatum at PND5. The surrounding chromogen deposition is due to positive neuropil staining, out of the plane of focus. D: GDNF immunostaining at PND14 is diffuse and faint. Scale bar = 10 µm.
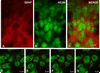
Fig. 4
Immunofluorescence labeling of GDNF and NeuN. A: A GDNF-positive striatal patch is identified by red AlexaFluor594 staining at PND6. Punctate and fibrillar staining is observed. B: Striatal neurons are identified by NeuN staining using a fluorescein label. C: A merged image reveals the NeuN-positive neurons enmeshed within the GDNF-positive fibrillar network. The extensive, overlapping GDNF fiber staining in this section observed by epifluorescence obscures any possible underlying GDNF staining within cytoplasm. Therefore, confocal analysis of serial optical sections was performed, as shown in consecutive sections D–G. These sections reveal fibrillar GDNF staining (arrowheads in E) and diffuse punctuate staining, some of it juxtaposed to neuronal cell bodies (arrows in F, G). No GDNF staining was observed in the cytoplasm of any NeuN-positive neurons in striosomes. The micron measurements in the lower right corner of panels D–G represent the Z-axis distances above the plane shown in D, arbitrarily defined as the initial plane at 1 µm. Scale bar = 10 µm in C (applies to A–G).
Fig. 5
Immunofluorescence labeling of GDNF and GFAP. Shown are three consecutive confocal planes, each labeled for GDNF (A,D,G) and GFAP (B,E,H) and merged (C,F,I) in a PND6 rat. The micron measurements in panels B, E, and H represent the Z-axis distances above the plane shown in H, arbitrarily defined as the initial plane at 1 µm. There is no cellular colocalization of the GDNF and GFAP staining. This is especially clear visually for the astrocyte fiber shown in panel H (arrow). Scale bar = 10 µm in I (applies to A–I).
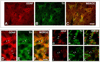
Fig. 6
Immunofluorescence labeling of GDNF and TH. A lower-power epifluorescence micrograph demonstrates a correspondence between striosomes identified by GDNF staining (A) and those identified by TH staining (B), as shown in the merged image (C). At higher power, epifluorescence demonstrates colocalization of GDNF (D) and TH (E) in striosomal fibers (merged in F) (examples of colocalization are indicated by pairs of white arrows). Colocalization is confirmed by confocal analysis. Two representative examples are shown in panel sets G–I and J–L. Scale bars = 100 µm in C (applies to A–C); 10 µm in F (applies to D–L).
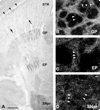
Fig. 7
Immunoperoxidase staining of GDNF in striatal efferent targets. A: A horizontal section immunoperoxidase stained for GDNF in PND6 rat. Faint striosomes (arrows) and the lateral crescent (arrowheads) can be observed in the striatum (STR). Distinct peroxidase staining is observed in globus pallidus (GP), entopeduncular nucleus (EP), and SNpr. At a cellular level, here shown in coronal sections by dark-field examination, numerous GDNF-positive fibers are observed in GP (B), EP (C), and SNpr (D). In each panel, examples of fibers are labeled with arrowheads. In none of these structures were cell bodies identified. Likewise, in SNpc, adjacent to the SNpr, cell bodies were not identified. Scale bars = 500 in A; 10 µm in D (applies to B–D).
Similar articles
- Anterograde axonal transport of AAV2-GDNF in rat basal ganglia.
Ciesielska A, Mittermeyer G, Hadaczek P, Kells AP, Forsayeth J, Bankiewicz KS. Ciesielska A, et al. Mol Ther. 2011 May;19(5):922-7. doi: 10.1038/mt.2010.248. Epub 2010 Nov 23. Mol Ther. 2011. PMID: 21102559 Free PMC article. - Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system.
Rosenblad C, Georgievska B, Kirik D. Rosenblad C, et al. Eur J Neurosci. 2003 Jan;17(2):260-70. doi: 10.1046/j.1460-9568.2003.02456.x. Eur J Neurosci. 2003. PMID: 12542662 - GDNF as a candidate striatal target-derived neurotrophic factor for the development of substantia nigra dopamine neurons.
Burke RE. Burke RE. J Neural Transm Suppl. 2006;(70):41-5. doi: 10.1007/978-3-211-45295-0_8. J Neural Transm Suppl. 2006. PMID: 17017507 Review. - Neuroprotection by neurotrophins and GDNF family members in the excitotoxic model of Huntington's disease.
Alberch J, Pérez-Navarro E, Canals JM. Alberch J, et al. Brain Res Bull. 2002 Apr;57(6):817-22. doi: 10.1016/s0361-9230(01)00775-4. Brain Res Bull. 2002. PMID: 12031278 Review.
Cited by
- How to Build and to Protect the Neuromuscular Junction: The Role of the Glial Cell Line-Derived Neurotrophic Factor.
Stanga S, Boido M, Kienlen-Campard P. Stanga S, et al. Int J Mol Sci. 2020 Dec 24;22(1):136. doi: 10.3390/ijms22010136. Int J Mol Sci. 2020. PMID: 33374485 Free PMC article. Review. - Treatment of Parkinson's disease with trophic factors.
Peterson AL, Nutt JG. Peterson AL, et al. Neurotherapeutics. 2008 Apr;5(2):270-80. doi: 10.1016/j.nurt.2008.02.003. Neurotherapeutics. 2008. PMID: 18394569 Free PMC article. Review. - The dual role of striatal interneurons: circuit modulation and trophic support for the basal ganglia.
Wegman E, Wosiski-Kuhn M, Luo Y. Wegman E, et al. Neural Regen Res. 2024 Jun 1;19(6):1277-1283. doi: 10.4103/1673-5374.382987. Epub 2023 Aug 14. Neural Regen Res. 2024. PMID: 37905876 Free PMC article. - Pitx3 is a critical mediator of GDNF-induced BDNF expression in nigrostriatal dopaminergic neurons.
Peng C, Aron L, Klein R, Li M, Wurst W, Prakash N, Le W. Peng C, et al. J Neurosci. 2011 Sep 7;31(36):12802-15. doi: 10.1523/JNEUROSCI.0898-11.2011. J Neurosci. 2011. PMID: 21900559 Free PMC article. - Growth Factors as Axon Guidance Molecules: Lessons From in vitro Studies.
Onesto MM, Short CA, Rempel SK, Catlett TS, Gomez TM. Onesto MM, et al. Front Neurosci. 2021 May 21;15:678454. doi: 10.3389/fnins.2021.678454. eCollection 2021. Front Neurosci. 2021. PMID: 34093120 Free PMC article. Review.
References
- Abe K, Hayashi T. Expression of the glial cell line-derived neurotrophic factor gene in rat brain after transient MCA occlusion. Brain Res. 1997;776:230–234. - PubMed
- Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain- derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–1716. - PMC - PubMed
- Bhattacharya B, Mandal C, Basu S, Sarkar PK. Regulation of alpha-and beta-tubulin mRNAs in rat brain during synaptogenesis. Brain Res. 1987;388:159–162. - PubMed
- Blum M, Weickert CS. GDNF mRNA expression in normal postnatal development, aging, and in weaver mutant mice. Neurobiol Aging. 1995;16:925–929. - PubMed
- Bowenkamp KE, Hoffman AF, Gerhardt GA, Henry MA, Biddle PT, Hoffer BJ, Granholm A-CE. Glial cell line-derived neurotrophic factor supports survival of injured midbrain dopaminergic neurons. J Comp Neurol. 1995;355:479–489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 NS026836/NS/NINDS NIH HHS/United States
- P50 NS038370/NS/NINDS NIH HHS/United States
- R01 NS026836-14/NS/NINDS NIH HHS/United States
- NS38370/NS/NINDS NIH HHS/United States
- R01 NS026836-13/NS/NINDS NIH HHS/United States
- R01 NS026836/NS/NINDS NIH HHS/United States
- P50 NS038370-07/NS/NINDS NIH HHS/United States
- NS26836/NS/NINDS NIH HHS/United States
- P50 NS038370-06/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous