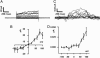Amphetamine induces dopamine efflux through a dopamine transporter channel - PubMed (original) (raw)
Amphetamine induces dopamine efflux through a dopamine transporter channel
Kristopher M Kahlig et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Drugs of abuse, including cocaine, amphetamine (AMPH), and heroin, elevate extracellular dopamine (DA) levels in the brain, thereby altering the activity/plasticity of reward circuits and precipitating addiction. The physiological release of DA occurs through the calcium-dependent fusion of a synaptic vesicle with the plasma membrane. Extracellular DA is cleared by uptake through the Na+/Cl- -dependent DA transporter (DAT). In contrast, the substrate AMPH induces nonvesicular release of DA mediated by DAT. Extracellular AMPH is generally believed to trigger DA efflux through DAT by facilitating exchange for cytosolic DA. Here, in outside-out patches from heterologous cells stably expressing DAT or from dopaminergic neurons, by using ionic conditions in the patch pipette that mimic those produced by AMPH stimulation, we report that AMPH causes DAT-mediated DA efflux by two independent mechanisms: (i) a slow process consistent with an exchange mechanism and (ii) a process that results in rapid (millisecond) bursts of DA efflux through a channel-like mode of DAT. Because channel-like release of DA induced by AMPH is rapid and contains a large number of DA molecules, with a single burst of DA on par with a quantum of DA from exocytotic release of a vesicle, this burst mode of release may play a role in the synaptic actions and psychostimulant properties of AMPH and related compounds. Unlike AMPH, the endogenous substrate DA, when present on both sides of the plasma membrane, inhibits this channel-like activity, thereby suggesting that the DAT channel-like mode cannot accumulate DA against a concentration gradient.
Figures
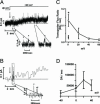
Fig. 1.
AMPH-induced hDAT-mediated DA efflux occurs via transporter-like and channel-like pathways. (A) Current trace of AMPH-induced hDAT transporter-like activity after a voltage step from -20 to +20 mV (see arrow). The AMPH-induced outward hDAT ionic current was defined as the current recorded in the presence of AMPH (10 μM) minus the current recorded after bath application of cocaine (10 μM) with AMPH still present. (Inset) DAT-mediated channel-like activity recorded from the same membrane patch (shown as asterisks). (B) Amperometric trace of AMPH-induced hDAT transporter-like DA efflux after a voltage step from -20 to +20 mV (see arrow, trace filtered at 2 Hz). The hDAT-mediated DA efflux was activated by bath application of 10 μM AMPH and was defined as the oxidation current recorded with AMPH minus the current recorded after bath application of cocaine (10 μM) with AMPH still present. (Inset) DAT-mediated channel-like efflux of DA (asterisks) recorded from the same membrane patch. (C) Transporter flux ratio of DA molecules to ions for voltage steps from 0 to +60 mV with 10 μM AMPH in the bath solution (□). We compared the flux ratio measured under control conditions (▪) (0 mV, no AMPH, cocaine subtracted) with that measured in the presence of AMPH. Data are shown as mean values ± SEM and were calculated 6–7 sec after the voltage step. *, = P < 0.05, by t test against control conditions. (D) Transporter-like efflux (DA molecules per sec) as measured in outside-out patches for voltage steps from -40 to +40 mV with 10 μM AMPH in the bath.
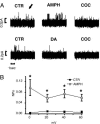
Fig. 2.
Substrate-regulated hDAT-mediated, outward channel-like activity. (A) Upper traces show sequential recordings of hDAT channel-like activity at +20 mV in control condition (CTR), after bath application of 10 μM AMPH (AMPH), and after bath application of 10 μM cocaine with AMPH still present (COC). Upward spikes signify outward channel-like current; see arrow. Lower traces show sequential recordings of hDAT channel-like activity at +20 mV in CTR, after bath application of 10 μM DA (DA), and after bath application of 10 μM cocaine with DA still present (COC). The fraction of the time that the hDAT channel is in the open state (NPo) and current (i) were calculated for each condition between 0 and 60 mV and reported in Fig. 2_B_ and Fig. 6, which is published as
supporting information
on the PNAS web site. (B) The hDAT-channel NPo increases after AMPH application, compared with the CTR condition for each voltage tested (n = 4–6 for each voltage). *, significantly different from CTR, P < 0.05, paired t test.
Fig. 3.
AMPH-induced DA effluxes and DAT currents in DA neurons. (A) DAT-mediated whole-cell currents recorded by stepping the membrane voltage from a holding potential of -60 mV to potentials between -100 and +100 mV. The internal solution of the whole-cell pipette contained 2 mM DA and 30 mM Na+. (C) Oxidation currents acquired concomitant to the whole-cell currents represented in A by voltage-clamping the cell between -100 and +100 mV with the whole-cell patch pipette. For voltage steps higher than -40 mV, the amperometric electrode recorded an oxidation current (positive) that increased during the voltage step, reaching a steady state. (B and D) Current (B) and amperometric (D) voltage relationships obtained from five different DA neurons. Data are presented as the mean ± SEM.
Fig. 4.
DA effluxes through an hDAT aqueous pore. The data shown are raw traces of channel-like activity and amperometric spikes; in each case, the channel activity was blocked at the end of the experiment by a 3-min application of cocaine to confirm that it was DAT-mediated. (A) hDAT-mediated channel activity (top trace, i) temporally correlates with discrete DA efflux events (bottom trace, a) at various voltages. All recordings were performed with 10 μM AMPH in the bath solution. The AMPH-induced hDAT channel-like efflux was blocked by bath solution of 10 μM cocaine with AMPH still present. To reduce background noise and highlight specific efflux events, the patch-clamp and amperometric recordings were filtered at 0.2 and 0.05 kHz, respectively. (B and C) Shown are recordings of individual efflux events generated by using excised membrane patches isolated from hDAT cells (C) or from excised membrane patches isolated from mouse DA neurons (B). Patch-clamp and amperometric recordings were filtered at 1 and 0.05 kHz, respectively. (D) The channel flux ratio between the number of DA molecules to ions that efflux when the hDAT channel opens is voltage-dependent in hDAT cells (□) and in DA neurons (○). The number of DA molecules and ions for each efflux event was calculated from the area under the respective discrete current events. Mean and SEM for 3–8 traces obtained from different experiments are shown.
Similar articles
- Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism.
Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A. Khoshbouei H, et al. J Biol Chem. 2003 Apr 4;278(14):12070-7. doi: 10.1074/jbc.M212815200. Epub 2003 Jan 29. J Biol Chem. 2003. PMID: 12556446 - Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux.
Binda F, Dipace C, Bowton E, Robertson SD, Lute BJ, Fog JU, Zhang M, Sen N, Colbran RJ, Gnegy ME, Gether U, Javitch JA, Erreger K, Galli A. Binda F, et al. Mol Pharmacol. 2008 Oct;74(4):1101-8. doi: 10.1124/mol.108.048447. Epub 2008 Jul 10. Mol Pharmacol. 2008. PMID: 18617632 Free PMC article. - Amphetamine-induced reverse transport of dopamine does not require cytosolic Ca2.
Støier JF, Konomi-Pilkati A, Apuschkin M, Herborg F, Gether U. Støier JF, et al. J Biol Chem. 2023 Aug;299(8):105063. doi: 10.1016/j.jbc.2023.105063. Epub 2023 Jul 18. J Biol Chem. 2023. PMID: 37468107 Free PMC article. - Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter.
Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Jones SR, et al. J Neurosci. 1998 Mar 15;18(6):1979-86. doi: 10.1523/JNEUROSCI.18-06-01979.1998. J Neurosci. 1998. PMID: 9482784 Free PMC article. Review. - Phosphorylation of the Amino Terminus of the Dopamine Transporter: Regulatory Mechanisms and Implications for Amphetamine Action.
Karam CS, Javitch JA. Karam CS, et al. Adv Pharmacol. 2018;82:205-234. doi: 10.1016/bs.apha.2017.09.002. Epub 2017 Oct 25. Adv Pharmacol. 2018. PMID: 29413521 Free PMC article. Review.
Cited by
- Dopaminergic and cholinergic modulation of striatal tyrosine hydroxylase interneurons.
Ibáñez-Sandoval O, Xenias HS, Tepper JM, Koós T. Ibáñez-Sandoval O, et al. Neuropharmacology. 2015 Aug;95:468-76. doi: 10.1016/j.neuropharm.2015.03.036. Epub 2015 Apr 20. Neuropharmacology. 2015. PMID: 25908399 Free PMC article. - Dynamic control of the dopamine transporter in neurotransmission and homeostasis.
Bu M, Farrer MJ, Khoshbouei H. Bu M, et al. NPJ Parkinsons Dis. 2021 Mar 5;7(1):22. doi: 10.1038/s41531-021-00161-2. NPJ Parkinsons Dis. 2021. PMID: 33674612 Free PMC article. Review. - Membrane-permeable C-terminal dopamine transporter peptides attenuate amphetamine-evoked dopamine release.
Rickhag M, Owens WA, Winkler MT, Strandfelt KN, Rathje M, Sørensen G, Andresen B, Madsen KL, Jørgensen TN, Wörtwein G, Woldbye DPD, Sitte H, Daws LC, Gether U. Rickhag M, et al. J Biol Chem. 2013 Sep 20;288(38):27534-27544. doi: 10.1074/jbc.M112.441295. Epub 2013 Jul 24. J Biol Chem. 2013. PMID: 23884410 Free PMC article. - HIV, Tat and dopamine transmission.
Gaskill PJ, Miller DR, Gamble-George J, Yano H, Khoshbouei H. Gaskill PJ, et al. Neurobiol Dis. 2017 Sep;105:51-73. doi: 10.1016/j.nbd.2017.04.015. Epub 2017 Apr 27. Neurobiol Dis. 2017. PMID: 28457951 Free PMC article. Review. - Currents in response to rapid concentration jumps of amphetamine uncover novel aspects of human dopamine transporter function.
Erreger K, Grewer C, Javitch JA, Galli A. Erreger K, et al. J Neurosci. 2008 Jan 23;28(4):976-89. doi: 10.1523/JNEUROSCI.2796-07.2008. J Neurosci. 2008. PMID: 18216205 Free PMC article.
References
- Giros, B. & Caron, M. G. (1993) Trends. Pharmacol. Sci. 14, 43-49. - PubMed
- Blakely, R. D., De Felice, L. J. & Hartzell, H. C. (1994) J. Exp. Biol. 196, 263-281. - PubMed
- Giros, B. (1996) Nature 379, 606-612. - PubMed
- Neal, M. J. & Iversen, L. L. (1972) Nat. New Biol. 235, 217-218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA13975/DA/NIDA NIH HHS/United States
- R01 MH058921/MH/NIMH NIH HHS/United States
- T32 MH065215/MH/NIMH NIH HHS/United States
- K02 MH057324/MH/NIMH NIH HHS/United States
- R01 EY015815/EY/NEI NIH HHS/United States
- R01 DA011495/DA/NIDA NIH HHS/United States
- DA14684/DA/NIDA NIH HHS/United States
- MH57324/MH/NIMH NIH HHS/United States
- EY015815/EY/NEI NIH HHS/United States
- MH58921/MH/NIMH NIH HHS/United States
- DA11495/DA/NIDA NIH HHS/United States
- R56 DA013975/DA/NIDA NIH HHS/United States
- R01 DA013975/DA/NIDA NIH HHS/United States
- R01 DA014684/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources