Activities of Pseudomonas aeruginosa effectors secreted by the Type III secretion system in vitro and during infection - PubMed (original) (raw)
Activities of Pseudomonas aeruginosa effectors secreted by the Type III secretion system in vitro and during infection
Vincent T Lee et al. Infect Immun. 2005 Mar.
Abstract
Pseudomonas aeruginosa utilizes a number of distinct pathways to secrete proteins that play various roles during infection. These include the type II secretion system, which is responsible for the secretion of the majority of exoproducts into the surrounding environment, including toxins and degradative enzymes. In contrast, the type III secretion system mediates the delivery of protein effectors directly into the cytoplasm of the host cell. Using tissue culture assays and a mouse acute-pneumonia model, we have determined the contribution of each of the type III effectors during infection. In strain PAK, ExoS is the major cytotoxin required for colonization and dissemination during infection. ExoT confers protection of tissue culture cells from type III-dependent lysis, while ExoY seemed to have little effect on cytotoxicity. ExoU is over 100-fold more cytotoxic than ExoS. The cytotoxicity of type II secretion was determined following deletion of the genes for the more toxic type III secretion system. The participation of these secretion systems during lifelong colonization of cystic fibrosis (CF) patients is unclear. By comparing clonal strains from the same patient isolated at the initial onset of P. aeruginosa infection and more than a decade later, after chronic colonization has been established, we show that initial strains are more cytotoxic than chronic strains that have evolved to reduce type III secretion. Constitutive expression of genes for the type III secretion system restored ExoS secretion but did not always reestablish cytotoxicity, suggesting that CF strains accumulate a number of mutations to reduce bacterial toxicity to the host.
Figures
FIG. 1.
Secretion profiles of P. aeruginosa strain PAK and isogenic derivatives induced for type III secretion. (A) Secreted proteins were separated by 12% PAGE and revealed by staining with Coomassie. The positions of ExoS, ExoT, and ExoY on the gel are indicated. WT, wild type. (B) Proteins were transferred onto PVDF membranes and detected with antibodies against ExoS (α-ExoS). In-frame deletion mutation of each exoenzyme gene resulted in the loss of that specific protein from the induced cultures.
FIG. 2.
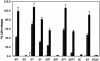
Contribution of each exoenzyme to the cytotoxicity of CHO cells infected with PAK or mutant derivatives. CHO cells were infected with PAK at a MOI of 10, and supernatants were collected at 3 (white bars), 4 (gray bars), and 5 h (black bars) postinfection. Cleared supernatants were analyzed for LDH release as a measure of cell lysis. WT, wild type.
FIG. 3.
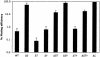
Survival of CHO cells after infection with PAK or mutant derivatives. CHO cells were infected with PAK at a MOI of 10 for 3 h. Cells were trypsinized, serially diluted, and seeded into media containing gentamicin. The foci that formed from CHO cells infected with various strains of PAK were enumerated. The relative plating efficiency was compared to that for uninfected cells, which was set to 100%. WT, wild type.
FIG. 4.
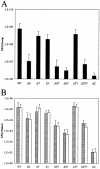
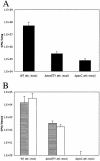
Colonization and dissemination of PAK in a mouse acute pneumonia model are attenuated in strains lacking exoS. Mice were inoculated intranasally with 5 × 107 CFU of PAK or derivatives. At 16 h postinfection, the lungs (A), 300 mg of livers (B; gray bars), and spleens (B; white bars) were harvested and the number of CFU of P. aeruginosa was determined by serial dilution and plating on L agar. Error bars indicate standard deviations.
FIG. 5.
ExoU secretion enhances cytotoxicity of PAK and Δ_STY_ strains. (A) Secreted proteins from low-calcium-induced cultures were separated by SDS-PAGE. Total proteins were revealed by staining with Coomassie. Proteins were transferred onto PVDF membranes and detected with antibodies against ExoS or ExoU. WT, wild type. (B) Plating efficiency of CHO cells after 2 h of infection with PAK strains harboring exoU.
FIG. 6.
Colonization of PAK exoU strains in vivo requires the presence of exoS, exoT, and exoY. Mice were inoculated intranasally with 2 × 106 CFU of PAK or derivatives. At 16 h postinfection, the lungs (A), 300 mg of livers (B; gray bars), and spleens (B; white bars) were harvested and the number of CFU of P. aeruginosa was determined by serial dilution and plating on L agar. Error bars indicate standard deviations. WT, wild type.
FIG. 7.
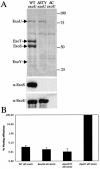
CF P. aeruginosa isolates lose the ability to secrete ExoS and induce cytotoxicity over the course of chronic colonization. (A) CHO cells were infected with PAK, PAKΔ_C_ (pscC deletion), PAKΔ_C_Δ_X_ (pscC and xcp operon deletions), or CF isolates at an initial MOI of 10 for either 4.5 (white bars) or 7.5 h (black bars). Cleared supernatants were analyzed for LDH release as a measure for cell lysis. (B) Secreted proteins from low-calcium-induced cultures were separated by SDS-PAGE. Proteins were transferred onto PVDF membranes and detected with antibodies against ExoS (α-ExoS). Patients were designated A to G; early strains are labeled 1, and late strains are labeled 2.
FIG. 8.
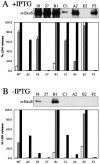
Overexpression of exsA results in the secretion of ExoS or ExoU in vitro but does not correlate with cytotoxicity to CHO cells. Secreted proteins from low-calcium-induced cultures were separated by SDS-PAGE. Proteins were transferred onto PVDF membranes and detected with antibodies against ExoS (α-ExoS) or ExoU. CHO cells were infected with PAK, PAKΔ_C_ (pscC deletion), or CF isolates harboring pMMB-exsA at an initial MOI of 10 for either 3 (white bars), 4.5 (gray bars), or 6 h (black bars). Cleared supernatants were analyzed for LDH release as a measure of cell lysis. (A) Cultures induced with IPTG. WT, wild type. (B) Culture without IPTG addition.
Similar articles
- Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung.
Shaver CM, Hauser AR. Shaver CM, et al. Infect Immun. 2004 Dec;72(12):6969-77. doi: 10.1128/IAI.72.12.6969-6977.2004. Infect Immun. 2004. PMID: 15557619 Free PMC article. - Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo.
Vance RE, Rietsch A, Mekalanos JJ. Vance RE, et al. Infect Immun. 2005 Mar;73(3):1706-13. doi: 10.1128/IAI.73.3.1706-1713.2005. Infect Immun. 2005. PMID: 15731071 Free PMC article. - The ADP-ribosyltransferase domain of the effector protein ExoS inhibits phagocytosis of Pseudomonas aeruginosa during pneumonia.
Rangel SM, Logan LK, Hauser AR. Rangel SM, et al. mBio. 2014 Jun 10;5(3):e01080-14. doi: 10.1128/mBio.01080-14. mBio. 2014. PMID: 24917597 Free PMC article. - Role of Pseudomonas aeruginosa type III effectors in disease.
Engel J, Balachandran P. Engel J, et al. Curr Opin Microbiol. 2009 Feb;12(1):61-6. doi: 10.1016/j.mib.2008.12.007. Epub 2009 Jan 23. Curr Opin Microbiol. 2009. PMID: 19168385 Review. - Immuno-modulatory functions of the type-3 secretion system and impacts on the pulmonary host defense: A role for ExoS of Pseudomonas aeruginosa in cystic fibrosis.
Belmadi N, Wu Y, Touqui L. Belmadi N, et al. Toxicon. 2018 Mar 1;143:68-73. doi: 10.1016/j.toxicon.2018.01.004. Epub 2018 Jan 12. Toxicon. 2018. PMID: 29339019 Review.
Cited by
- The Pseudomonas aeruginosa PhoP-PhoQ two-component regulatory system is induced upon interaction with epithelial cells and controls cytotoxicity and inflammation.
Gellatly SL, Needham B, Madera L, Trent MS, Hancock RE. Gellatly SL, et al. Infect Immun. 2012 Sep;80(9):3122-31. doi: 10.1128/IAI.00382-12. Epub 2012 Jun 18. Infect Immun. 2012. PMID: 22710876 Free PMC article. - During bacteremia, Pseudomonas aeruginosa PAO1 adapts by altering the expression of numerous virulence genes including those involved in quorum sensing.
Beasley KL, Cristy SA, Elmassry MM, Dzvova N, Colmer-Hamood JA, Hamood AN. Beasley KL, et al. PLoS One. 2020 Oct 15;15(10):e0240351. doi: 10.1371/journal.pone.0240351. eCollection 2020. PLoS One. 2020. PMID: 33057423 Free PMC article. - Host response and bacterial virulence factor expression in Pseudomonas aeruginosa and Streptococcus pneumoniae corneal ulcers.
Karthikeyan RS, Priya JL, Leal SM Jr, Toska J, Rietsch A, Prajna V, Pearlman E, Lalitha P. Karthikeyan RS, et al. PLoS One. 2013 Jun 4;8(6):e64867. doi: 10.1371/journal.pone.0064867. Print 2013. PLoS One. 2013. PMID: 23750216 Free PMC article. - Catheter-associated urinary tract infection by Pseudomonas aeruginosa progresses through acute and chronic phases of infection.
Mekonnen SA, El Husseini N, Turdiev A, Carter JA, Belew AT, El-Sayed NM, Lee VT. Mekonnen SA, et al. Proc Natl Acad Sci U S A. 2022 Dec 13;119(50):e2209383119. doi: 10.1073/pnas.2209383119. Epub 2022 Dec 5. Proc Natl Acad Sci U S A. 2022. PMID: 36469780 Free PMC article. - RNA-Seq Transcriptomic Responses of Full-Thickness Dermal Excision Wounds to Pseudomonas aeruginosa Acute and Biofilm Infection.
Karna SL, D'Arpa P, Chen T, Qian LW, Fourcaudot AB, Yamane K, Chen P, Abercrombie JJ, You T, Leung KP. Karna SL, et al. PLoS One. 2016 Oct 28;11(10):e0165312. doi: 10.1371/journal.pone.0165312. eCollection 2016. PLoS One. 2016. PMID: 27792773 Free PMC article.
References
- Bally, M., A. Filloux, M. Akrim, G. Ball, A. Lazdunski, and J. Tommassen. 1992. Protein secretion in Pseudomonas aeruginosa: characterization of seven xcp genes and processing of secretory apparatus components by prepilin peptidase. Mol. Microbiol. 6:1121-1131. - PubMed
- Banwart, B., M. L. Splaingard, P. M. Farrell, M. J. Rock, P. L. Havens, J. Moss, M. E. Ehrmantraut, D. W. Frank, and J. T. Barbieri. 2002. Children with cystic fibrosis produce an immune response against exoenzyme S, a type III cytotoxin of Pseudomonas aeruginosa. J. Infect. Dis. 185:269-270. - PubMed
- Dacheux, D., J. Goure, J. Chabert, Y. Usson, and I. Attree. 2001. Pore-forming activity of type III system-secreted proteins leads to oncosis of Pseudomonas aeruginosa-infected macrophages. Mol. Microbiol. 40:76-85. - PubMed
- Feltman, H., G. Schulert, S. Khan, M. Jain, L. Peterson, and A. R. Hauser. 2001. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147:2659-2669. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical