Amino acids 1055 to 1192 in the S2 region of severe acute respiratory syndrome coronavirus S protein induce neutralizing antibodies: implications for the development of vaccines and antiviral agents - PubMed (original) (raw)
. 2005 Mar;79(6):3289-96.
doi: 10.1128/JVI.79.6.3289-3296.2005.
Aihua Zhang, Shuo Shen, Kuo-Ming Lip, Burtram C Fielding, Timothy H P Tan, Chih-Fong Chou, Chay Boon Loh, Sifang Wang, Jianlin Fu, Xiaoming Yang, Seng Gee Lim, Wanjin Hong, Yee-Joo Tan
Affiliations
- PMID: 15731223
- PMCID: PMC1075733
- DOI: 10.1128/JVI.79.6.3289-3296.2005
Amino acids 1055 to 1192 in the S2 region of severe acute respiratory syndrome coronavirus S protein induce neutralizing antibodies: implications for the development of vaccines and antiviral agents
Choong-Tat Keng et al. J Virol. 2005 Mar.
Abstract
The spike (S) protein of the severe acute respiratory syndrome coronavirus (SARS-CoV) interacts with cellular receptors to mediate membrane fusion, allowing viral entry into host cells; hence it is recognized as the primary target of neutralizing antibodies, and therefore knowledge of antigenic determinants that can elicit neutralizing antibodies could be beneficial for the development of a protective vaccine. Here, we expressed five different fragments of S, covering the entire ectodomain (amino acids 48 to 1192), as glutathione S-transferase fusion proteins in Escherichia coli and used the purified proteins to raise antibodies in rabbits. By Western blot analysis and immunoprecipitation experiments, we showed that all the antibodies are specific and highly sensitive to both the native and denatured forms of the full-length S protein expressed in virus-infected cells and transfected cells, respectively. Indirect immunofluorescence performed on fixed but unpermeabilized cells showed that these antibodies can recognize the mature form of S on the cell surface. All the antibodies were also able to detect the maturation of the 200-kDa form of S to the 210-kDa form by pulse-chase experiments. When the antibodies were tested for their ability to inhibit SARS-CoV propagation in Vero E6 culture, it was found that the anti-SDelta10 antibody, which was targeted to amino acid residues 1029 to 1192 of S, which include heptad repeat 2, has strong neutralizing activities, suggesting that this region of S carries neutralizing epitopes and is very important for virus entry into cells.
Figures
FIG. 1.
Schematic diagram showing the different regions of S encoded by the plasmids used in this study. (Top) Regions (arrows) representing the five bacterially expressed proteins (SΔ1, SΔ2, SΔ3, SΔ9, and SΔ10) used to raise the antibodies. The numbers at each end of the arrows represent the positions of the amino acid residues in the S protein. α, anti. (Bottom) Full-length S and the S proteins with C-terminal deletions (SΔ11 to SΔ16) were expressed in Cos7 cells. Numbers at the end of the proteins represent the positions of amino acid residues of the respective proteins. Heptad repeat regions HR1 (aa 891 to 975) and HR2 (aa 1148 to 1193) are shown.
FIG. 2.
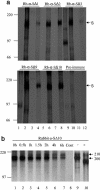
Western blot analysis for detection of the S protein. Cos7 cells were transfected with plasmid pKT-S (lanes 1, 3, 5, 7, 9, 11, 13, 15, 17, 19, 21, and 23) or with a plasmid without an insert as a negative control (lanes 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24). Cell lysates were separated in 10% PAGE gel. Western blotting was performed with the antisera, preimmune sera, and control serum indicated on top of each gel. Arrowheads (left), molecular masses (in kilodaltons) of specific S proteins. Membranes were reprobed with mouse anti-β-actin as the loading control. High-range Rainbow molecular weight markers (right; Amersham) were used. α, anti.
FIG. 3.
Radiolabeled immunoprecipitation. Cos7 cells were transfected with plasmids expressing full-length S and the C-terminally truncated S proteins, SΔ11, SΔ12, SΔ13, SΔ14, SΔ15, and SΔ16 (lanes 1, 2, 3, 4, 5, 6, and 7) or with a plasmid without an insert as a negative control (lanes 8). 35S-labeled S proteins were immunoprecipitated with the antibodies indicated (top of each autoradiograph) and then separated in SDS-7.5% PAGE gels. Arrowheads (left), molecular masses (in kilodaltons) of specific S proteins. High-range Rainbow molecular weight markers (right; Amersham) were used. α, anti.
FIG. 4.
(a) Immunoprecipitation of the S protein in cell lysate and medium of virus-infected Vero E6 cells. Vero E6 cells in 60-mm dishes were infected (lanes 2, 4, 6, 8, 10, and 12) with SARS-CoV at a MOI of 5 or mock infected (lanes 1, 3, 5, 7, 9, and 11). Cells were radiolabeled 5 h postinfection with [35S]Met-Cys for 1 h. Cells were chased with medium containing 4 mM Met-Cys for 3 h. Cells (lanes 1, 2, 5, 6, 9, and 10) and media (lanes 3, 4, 7, 8, 11, and 12) were harvested with 1× or 1/5 volume of 5× lysis buffer, respectively. Samples were immunoprecipitated with antisera and preimmune serum as indicated and then separated in SDS-7.5% PAGE gels. The S-specific bands are indicated on the right. High-range Rainbow molecular weight markers (left; Amersham) were used. Rb, rabbit; α, anti. (b) Time-course of S protein maturation. Cos7 cells transfected with pKT-S were radiolabeled and chased for 0, 0.5, 1, 2, 4, and 6 h (lanes 1, 2, 3, 4, 5, 6, and 7). Cos7 cells transfected with a plasmid without an insert were harvested at 6 h as a negative control (lane 8). All the cell lysates were immunoprecipitated with rabbit anti-SΔ10 antibodies and then separated in SDS-7.5% PAGE gels. In a separate experiment, the immunoprecipitated proteins (6 h posttransfection) were treated (+) with EndoH (lane 10) or untreated (−) as a control (lane 9). The S-specific bands and their molecular masses (in kilodaltons) are indicated on the right. High-range Rainbow molecular weight markers (left; Amersham) were used.
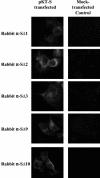
FIG. 5.
S protein expressed on the surfaces of transiently transfected Cos7 cells. (Left column) Cos7 cells were transfected with full-length S and visualized under UV light. The green fluorescence represents the S protein expressed on the surfaces of Cos7 cells probed with primary antibodies rabbit anti-SΔ1 (α-SΔ1), anti-SΔ2, anti-SΔ3, anti-SΔ9, and anti-SΔ10, followed by fluorescein isothiocyanate-conjugated secondary antibodies. (Right column) Cos7 cells were mock transfected with the empty vector and probed with the antibodies indicated on the left, as control experiments, and visualized under UV light.
Similar articles
- An exposed domain in the severe acute respiratory syndrome coronavirus spike protein induces neutralizing antibodies.
Zhou T, Wang H, Luo D, Rowe T, Wang Z, Hogan RJ, Qiu S, Bunzel RJ, Huang G, Mishra V, Voss TG, Kimberly R, Luo M. Zhou T, et al. J Virol. 2004 Jul;78(13):7217-26. doi: 10.1128/JVI.78.13.7217-7226.2004. J Virol. 2004. PMID: 15194798 Free PMC article. - SARS patients-derived human recombinant antibodies to S and M proteins efficiently neutralize SARS-coronavirus infectivity.
Liang MF, Du RL, Liu JZ, Li C, Zhang QF, Han LL, Yu JS, Duan SM, Wang XF, Wu KX, Xiong ZH, Jin Q, Li DX. Liang MF, et al. Biomed Environ Sci. 2005 Dec;18(6):363-74. Biomed Environ Sci. 2005. PMID: 16544518 - Vaccine design for severe acute respiratory syndrome coronavirus.
He Y, Jiang S. He Y, et al. Viral Immunol. 2005;18(2):327-32. doi: 10.1089/vim.2005.18.327. Viral Immunol. 2005. PMID: 16035944 Review. - Neutralizing human monoclonal antibodies to severe acute respiratory syndrome coronavirus: target, mechanism of action, and therapeutic potential.
Coughlin MM, Prabhakar BS. Coughlin MM, et al. Rev Med Virol. 2012 Jan;22(1):2-17. doi: 10.1002/rmv.706. Epub 2011 Sep 8. Rev Med Virol. 2012. PMID: 21905149 Free PMC article. Review.
Cited by
- SARS-CoV-2: An Update on Potential Antivirals in Light of SARS-CoV Antiviral Drug Discoveries.
Elshabrawy HA. Elshabrawy HA. Vaccines (Basel). 2020 Jun 23;8(2):335. doi: 10.3390/vaccines8020335. Vaccines (Basel). 2020. PMID: 32585913 Free PMC article. Review. - The severe acute respiratory syndrome coronavirus 3a is a novel structural protein.
Shen S, Lin PS, Chao YC, Zhang A, Yang X, Lim SG, Hong W, Tan YJ. Shen S, et al. Biochem Biophys Res Commun. 2005 Apr 29;330(1):286-92. doi: 10.1016/j.bbrc.2005.02.153. Biochem Biophys Res Commun. 2005. PMID: 15781262 Free PMC article. - Severe acute respiratory syndrome coronavirus protein 7a interacts with hSGT.
Fielding BC, Gunalan V, Tan TH, Chou CF, Shen S, Khan S, Lim SG, Hong W, Tan YJ. Fielding BC, et al. Biochem Biophys Res Commun. 2006 May 19;343(4):1201-8. doi: 10.1016/j.bbrc.2006.03.091. Epub 2006 Mar 24. Biochem Biophys Res Commun. 2006. PMID: 16580632 Free PMC article. - Pandemics and the English Language: Concepts Critical for Conversing About COVID-19.
Greenspan NS, Pereda GA. Greenspan NS, et al. Pathog Immun. 2022 Nov 10;7(2):78-92. doi: 10.20411/pai.v7i2.542. eCollection 2022. Pathog Immun. 2022. PMID: 36407560 Free PMC article. - SARS-CoV spike protein-expressing recombinant vaccinia virus efficiently induces neutralizing antibodies in rabbits pre-immunized with vaccinia virus.
Kitabatake M, Inoue S, Yasui F, Yokochi S, Arai M, Morita K, Shida H, Kidokoro M, Murai F, Le MQ, Mizuno K, Matsushima K, Kohara M. Kitabatake M, et al. Vaccine. 2007 Jan 8;25(4):630-7. doi: 10.1016/j.vaccine.2006.08.039. Epub 2006 Sep 11. Vaccine. 2007. PMID: 17011679 Free PMC article.
References
- Bosch, B. J., B. E. E. Martina, R. Van Der Zee, J. Lepault, B. J. Haijema, C. Versluis, A. J. Heck, R. De Groot, A. D. M. E. Osterhaus, and P. J. M. Rottier. 2004. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc. Natl. Acad. Sci. USA 101:8455-8460. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous