The influenza A virus M2 cytoplasmic tail is required for infectious virus production and efficient genome packaging - PubMed (original) (raw)
The influenza A virus M2 cytoplasmic tail is required for infectious virus production and efficient genome packaging
Matthew F McCown et al. J Virol. 2005 Mar.
Abstract
The M2 integral membrane protein encoded by influenza A virus possesses an ion channel activity that is required for efficient virus entry into host cells. The role of the M2 protein cytoplasmic tail in virus replication was examined by generating influenza A viruses encoding M2 proteins with truncated C termini. Deletion of 28 amino acids (M2Stop70) resulted in a virus that produced fourfold-fewer particles but >1,000-fold-fewer infectious particles than wild-type virus. Expression of the full-length M2 protein in trans restored the replication of the M2 truncated virus. Although the M2Stop70 virus particles were similar to wild-type virus in morphology, the M2Stop70 virions contained reduced amounts of viral nucleoprotein and genomic RNA, indicating a defect in vRNP packaging. The data presented indicate the M2 cytoplasmic tail plays a role in infectious virus production by coordinating the efficient packaging of genome segments into influenza virus particles.
Figures
FIG. 1.
Effect of M2 cytoplasmic tail truncations on protein stability and ion channel activity. (A) 293T cells expressing the indicated M2 protein were metabolically labeled, and cell lysates were immunoprecipitated with an MAb to M2. The proteins were separated by SDS-PAGE and visualized by using a phosphorimager. (B) 293T cells transfected with pC FPV HA and pC, pC M2, or pC M2Stop70 were metabolically labeled, cell lysates were generated at the indicated times, and immunoprecipitations were performed with polyclonal sera against FPV HA. The proteins were separated by SDS-PAGE and visualized with a phosphorimager. The amount of labeled HA2, for each sample, after a 120-min chase was normalized to the pC sample without amantadine.
FIG. 2.
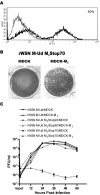
Effect of M2 cytoplasmic tail truncations on influenza A virus replication. (A) MDCK-M2 cells were analyzed by flow cytometry for expression of the M2 protein. The thin line represents cells stained with only secondary antibody, and the thick line represents cells stained with a primary antibody against M2 and a fluorescein isothiocyanate-conjugated secondary antibody. (B) A plaque assay on the virus rWSN M-Ud M2Stop70 was performed with MDCK and MDCK-M2 cells. (C) A multistep growth curve was performed on MDCK and MDCK-M2 cells with the viruses rWSN M-Ud, rWSN M-Ud M2Stop90, and rWSN M-Ud M2Stop70.
FIG. 3.
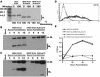
The M2Stop70 protein is unable to support influenza A virus replication. (A) 293T cells were infected at an MOI of 1.0 with the indicated virus and metabolically labeled, and cell lysates were immunoprecipitated with an MAb to M2. The proteins were separated by SDS-PAGE and visualized with a phosphorimager. (B and C) 293T (B) or MDCK (C) cells were infected at an MOI of 1.0 with the indicated virus. At 3, 6, 9, and 12 h postinfection the cells were lysed, and the polypeptides were separated by SDS-PAGE and analyzed by Western blotting for the M1 and M2 proteins. (D) MDCK-M2Stop70 cells were analyzed by flow cytometry for expression of the M2 protein. The thin line represents cells stained with only secondary antibody, and the thick line represents cells stained with a primary antibody against M2 and a fluorescein isothiocyanate-conjugated secondary antibody. (E) A multistep growth curve was performed on MDCK and MDCK-M2Stop70 cells with rWSN M-Ud and rWSN M-Ud M2Stop70.
FIG. 4.
The effect of M2 protein _trans_-complementation on rWSN M-Udorn M2Stop70 virus morphology and polypeptide composition. (A) Purified virions were negatively stained with uranyl acetate and examined with a JEOL 1200EX transmission electron microscope. Scale bar, 100 nm. (B and C) Purified virions from MDCK, MDCK-M2, and MDCK-M2Stop70 infected cells were analyzed by Western blotting. In panel B an MAb to M2 was used, and in panel C antibodies to HA, NP, and M1 were used. The band migrating slightly faster than full-length M2 is a proteolytic fragment of M2.
FIG. 5.
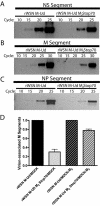
RNA incorporation into rWSN M-Ud and rWSN M-Ud M2Stop70 virions. RNA was extracted from the indicated virions, and a semiquantitative RT-PCR was performed with primers specific for the NS segment (A), the M segment (B), and the NP segment (C). (D) RNA was extracted from the indicated virions, reverse transcribed with primers specific for the M segment, and quantitated by real-time PCR.
FIG. 6.
Characterization of MDCK cells with inducible M2 expression. MDCK-Tet M2 cells were incubated in the absence or presence of increasing concentrations of doxycycline for 48 h. At 48 h the cells were lysed, the polypeptides were separated by SDS-PAGE and analyzed by Western blotting with an antibody to the M2 protein.
FIG. 7.
Analysis of rWSN M-Ud M2Stop70 Revertant. (A) Western blot of virus-infected cell lysates with MAbs to M2 and M1. (B) A multistep growth curve was performed on MDCK cells infected with rWSN M-Ud, rWSN M-Ud M2Stop70, or rWSN M-Ud M2Stop70 revertant.
Similar articles
- Distinct domains of the influenza a virus M2 protein cytoplasmic tail mediate binding to the M1 protein and facilitate infectious virus production.
McCown MF, Pekosz A. McCown MF, et al. J Virol. 2006 Aug;80(16):8178-89. doi: 10.1128/JVI.00627-06. J Virol. 2006. PMID: 16873274 Free PMC article. - Mutations in the Influenza A Virus M1 Protein Enhance Virus Budding To Complement Lethal Mutations in the M2 Cytoplasmic Tail.
Liu H, Grantham ML, Pekosz A. Liu H, et al. J Virol. 2017 Dec 14;92(1):e00858-17. doi: 10.1128/JVI.00858-17. Print 2018 Jan 1. J Virol. 2017. PMID: 29046451 Free PMC article. - Influenza a virus M2 ion channel activity is essential for efficient replication in tissue culture.
Takeda M, Pekosz A, Shuck K, Pinto LH, Lamb RA. Takeda M, et al. J Virol. 2002 Feb;76(3):1391-9. doi: 10.1128/jvi.76.3.1391-1399.2002. J Virol. 2002. PMID: 11773413 Free PMC article. - [Structure and function of the influenza virus M2 ion channel protein].
Sakaguchi T. Sakaguchi T. Nihon Rinsho. 1997 Oct;55(10):2587-92. Nihon Rinsho. 1997. PMID: 9360376 Review. Japanese. - Packaging signal of influenza A virus.
Li X, Gu M, Zheng Q, Gao R, Liu X. Li X, et al. Virol J. 2021 Feb 17;18(1):36. doi: 10.1186/s12985-021-01504-4. Virol J. 2021. PMID: 33596956 Free PMC article. Review.
Cited by
- Conserved sequence features in intracellular domains of viral spike proteins.
Ngo VN, Winski DP, Aho B, Kamath PL, King BL, Waters H, Zimmerberg J, Sodt A, Hess ST. Ngo VN, et al. Virology. 2024 Nov;599:110198. doi: 10.1016/j.virol.2024.110198. Epub 2024 Aug 2. Virology. 2024. PMID: 39116647 - Antibody inhibition of influenza A virus assembly and release.
He Y, Guo Z, Subiaur S, Benegal A, Vahey MD. He Y, et al. J Virol. 2024 Feb 20;98(2):e0139823. doi: 10.1128/jvi.01398-23. Epub 2024 Jan 5. J Virol. 2024. PMID: 38179944 Free PMC article. - The M2 proteins of bat influenza A viruses reveal atypical features compared to conventional M2 proteins.
Thompson D, Cismaru CV, Rougier JS, Schwemmle M, Zimmer G. Thompson D, et al. J Virol. 2023 Aug 31;97(8):e0038823. doi: 10.1128/jvi.00388-23. Epub 2023 Aug 4. J Virol. 2023. PMID: 37540019 Free PMC article. - 2019-2020 H1N1 clade A5a.1 viruses have better in vitro fitness compared with the co-circulating A5a.2 clade.
Swanson NJ, Marinho P, Dziedzic A, Jedlicka A, Liu H, Fenstermacher K, Rothman R, Pekosz A. Swanson NJ, et al. Sci Rep. 2023 Jun 23;13(1):10223. doi: 10.1038/s41598-023-37122-z. Sci Rep. 2023. PMID: 37353648 Free PMC article. - 2019-20 H1N1 clade A5a.1 viruses have better in vitro replication compared with the co-circulating A5a.2 clade.
Swanson NJ, Marinho P, Dziedzic A, Jedlicka A, Liu H, Fenstermacher K, Rothman R, Pekosz A. Swanson NJ, et al. bioRxiv [Preprint]. 2023 Feb 26:2023.02.26.530085. doi: 10.1101/2023.02.26.530085. bioRxiv. 2023. PMID: 36865250 Free PMC article. Updated. Preprint.
References
- Arzt, S., F. Baudin, A. Barge, P. Timmins, W. P. Burmeister, and R. W. H. Ruigrok. 2001. Combined results from solution studies on intact influenza virus M1 protein and from a new crystal form of its N-terminal domain show that M1 is an elongated monomer. Virology 279:439-446. - PubMed
- Bukrinskaya, A. G., N. K. Vorkunova, G. V. Kornilayeva, R. A. Narmanbetova, and G. K. Vorkunova. 1982. Influenza virus uncoating in infected cells and effect of rimantadine. J. Gen. Virol. 60:49-59. - PubMed
- Bukrinskaya, A. G., N. K. Vorkunova, and N. L. Pushkarskaya. 1982. Uncoating of a rimantadine-resistant variant of influenza virus in the presence of rimantadine. J. Gen. Virol. 60:61-66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources