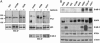ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines - PubMed (original) (raw)
ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines
Jeffrey A Engelman et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Therapies that target the EGF receptor (EGFR), such as gefitinib (IRESSA), are effective in a subset of patients with advanced non-small cell lung cancer (NSCLC). The differences in intracellular signaling networks between gefitinib-sensitive and -resistant NSCLCs remain poorly understood. In this study, we observe that gefitinib reduces phospho-Akt levels only in NSCLC cell lines in which it inhibits growth. To elucidate the mechanism underlying this observation, we compared immunoprecipitates of phosphoinositide 3-kinase (PI3K) between gefitinib-sensitive and -resistant NSCLC cell lines. We observe that PI3K associates with ErbB-3 exclusively in gefitinib-sensitive NSCLC cell lines. Gefitinib dissociates this complex, thereby linking EGFR inhibition to decreased Akt activity. In contrast, gefitinib-resistant cells do not use ErbB-3 to activate the PI3K/Akt pathway. In fact, abundant ErbB-3 expression is detected only in gefitinib-sensitive NSCLC cell lines. Two gefitinib-sensitive NSCLC cell lines with endogenous distinct activating EGFR mutations (L858R and Del747-749), frequently observed in NSCLC patients who respond to gefitinib, also use ErbB-3 to couple to PI3K. Down-regulation of ErbB-3 by means of short hairpin RNA leads to decreased phospho-Akt levels in the gefitinib-sensitive NSCLC cell lines, Calu-3 (WT EGFR) and H3255 (L858R EGFR), but has no effect on Akt activation in the gefitinib-resistant cell lines, A549 and H522. We conclude that ErbB-3 is used to couple EGFR to the PI3K/Akt pathway in gefitinib-sensitive NSCLC cell lines harboring WT and mutant EGFRs.
Figures
Fig. 1.
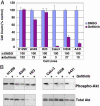
Akt activity is inhibited by gefitinib exclusively in NSCLC cell lines whose growth is inhibited by gefitinib. (A) Growth assays were performed on the cell lines shown while incubated in the presence or absence of gefitinib. Approximately 25,000–40,000 cells were seeded in 12-well plates and propagated in full serum (10% FBS) in the presence of gefitinib (1 μM) or vehicle control (DMSO). Cells were counted on day 5. Results are expressed as a percentage of control. Numerical depiction of the results is displayed below the graph. (B) Cell lines were grown in the presence of gefitinib (1 μM) or vehicle control (DMSO) for 6 h. Fifty micrograms of extract was probed with an antibody against phospho-Akt (Ser-473) and total Akt.
Fig. 2.
PI3K regulatory subunit p85 binds ErbB-3 in a gefitinib-dependent manner exclusively in NSCLC cell lines sensitive to gefitinib. (A) Three NSCLC cell lines resistant to gefitinib (H23, A549, and H1299) and three cell lines sensitive to gefitinib (H358, Calu-3, and A431) were grown in the presence or absence of gefitinib (1 μM) for 6 h. Extracts (≈1 mg) were immunoprecipitated with an anti-p85 antibody and subjected to immunoblot assay with anti-PTyr, anti-p85, and anti-ErbB-3 antibodies. * marks a PTyr protein observed in p85 coprecipitations in gefitinib-sensitive cells whose association is significantly diminished in the presence of gefitinib. (B) 50 micrograms of extracts from the cell lines shown were probed with antibodies against ErbB-3, EGFR, ErbB-2, PTEN, and β-actin. * denotes cells that have an IC50 of <1 μM to gefitinib (gefitinib-sensitive). Note that H322 and H522 cells are resistant to 1 μM gefitinib (data not shown).
Fig. 3.
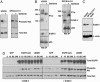
EGF stimulates PI3K association with ErbB-3 in a gefitinib-sensitive NSCLC cell line. (A) Calu-3 cells were incubated in propagation medium in the presence or absence of gefitinib (1 μM) for 6 h followed by stimulation with or without EGF (60 ng/ml) for 10 min. Extracts were immunoprecipitated with anti-p85 antibodies and then subjected to immunoblot analysis with antibodies against PTyr, ErbB-3, and p85. (B) Calu-3 cells were exposed or not exposed to gefitinib for 3 h. Extracts were immunoprecipitated with anti-ErbB-3 antibodies and subjected to immunoblot analysis with anti-PTyr, anti-ErbB-3, and anti-p85 antibodies. (C) Calu-3 cells were incubated in the presence of different concentrations of gefitinib (0, 0.25, 1, and 4 μM). Cell extracts were prepared, and anti-p85 IPs were performed as in Fig. 2. IPs were probed with antibodies against ErbB-3, PTyr, and p85. Fifty micrograms of extracts prepared from these Calu-3 cells were probed with the indicated antibodies.
Fig. 4.
NSCLC cell lines harboring activating EGFR mutations demonstrate a gefitinib-labile interaction between p85 and ErbB-3. (A) H3255 cells (L858R) and DFCILU-011 cells (Del747-749) were grown in the presence of gefitinib (1 μM) or vehicle control (DMSO) for 6 h. Fifty micrograms of extract was probed with an antibody against phospho-Akt (Ser-473) and total Akt. (B) The cells were treated exactly as in A. Extracts (≈1 mg) were immunoprecipitated with an anti-p85 antibody and subjected to immunoblot assay with anti-PTyr, anti-p85, and anti-ErbB-3 antibodies. (C) Total extracts (50 μg) were probed with antibodies specific for ErbB-3 and β-actin. Equal amounts of extracts for A549 cells were included for control. (D) CHO cells were transiently transfected with EGFR (WT), EGFR L858R, or GFP control with either ErbB-3 or GFP control. About 30 h after transfection, cells were serum-starved overnight, followed by stimulation with EGF (60 ng/ml) for the indicated number of minutes (0, 10, 60, or 240). Cells were lysed, and 40 μg of extracts were assessed for expression of EGFR, phospho-Akt, and total Akt. SDS/PAGE gels from the -ErbB-3 and +ErbB-3 conditions were treated identically, incubated together in primary and secondary antibody solutions, and developed together on the same piece of film.
Fig. 5.
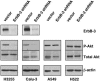
ErbB-3 shRNA down-regulates phospho-Akt levels in the gefitinib-sensitive cell lines, H3255 and Calu-3. The Calu-3 and H3255 cell lines were infected with ErbB-3 shRNA or control lentiviral vector. Five days after infection, protein extracts were prepared and probed with antibodies against ErbB-3, phospho-Akt (Ser-473), total Akt, and β-actin. The A549 and H522 cell lines, which do not express ErbB-3 and are not sensitive to gefitinib, were infected by identical methods to serve as controls.
Similar articles
- Gefitinib induces apoptosis in the EGFRL858R non-small-cell lung cancer cell line H3255.
Tracy S, Mukohara T, Hansen M, Meyerson M, Johnson BE, Jänne PA. Tracy S, et al. Cancer Res. 2004 Oct 15;64(20):7241-4. doi: 10.1158/0008-5472.CAN-04-1905. Cancer Res. 2004. PMID: 15492241 - Gefitinib (IRESSA) sensitive lung cancer cell lines show phosphorylation of Akt without ligand stimulation.
Noro R, Gemma A, Kosaihira S, Kokubo Y, Chen M, Seike M, Kataoka K, Matsuda K, Okano T, Minegishi Y, Yoshimura A, Kudoh S. Noro R, et al. BMC Cancer. 2006 Dec 6;6:277. doi: 10.1186/1471-2407-6-277. BMC Cancer. 2006. PMID: 17150102 Free PMC article. - HER2 overexpression increases sensitivity to gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, through inhibition of HER2/HER3 heterodimer formation in lung cancer cells.
Hirata A, Hosoi F, Miyagawa M, Ueda S, Naito S, Fujii T, Kuwano M, Ono M. Hirata A, et al. Cancer Res. 2005 May 15;65(10):4253-60. doi: 10.1158/0008-5472.CAN-04-2748. Cancer Res. 2005. PMID: 15899817 - Preclinical rationale for PI3K/Akt/mTOR pathway inhibitors as therapy for epidermal growth factor receptor inhibitor-resistant non-small-cell lung cancer.
Gadgeel SM, Wozniak A. Gadgeel SM, et al. Clin Lung Cancer. 2013 Jul;14(4):322-32. doi: 10.1016/j.cllc.2012.12.001. Epub 2013 Jan 16. Clin Lung Cancer. 2013. PMID: 23332287 Review.
Cited by
- Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors.
Jia Y, Yun CH, Park E, Ercan D, Manuia M, Juarez J, Xu C, Rhee K, Chen T, Zhang H, Palakurthi S, Jang J, Lelais G, DiDonato M, Bursulaya B, Michellys PY, Epple R, Marsilje TH, McNeill M, Lu W, Harris J, Bender S, Wong KK, Jänne PA, Eck MJ. Jia Y, et al. Nature. 2016 Jun 2;534(7605):129-32. doi: 10.1038/nature17960. Epub 2016 May 25. Nature. 2016. PMID: 27251290 Free PMC article. - Diminished functional role and altered localization of SHP2 in non-small cell lung cancer cells with EGFR-activating mutations.
Furcht CM, Muñoz Rojas AR, Nihalani D, Lazzara MJ. Furcht CM, et al. Oncogene. 2013 May 2;32(18):2346-55, 2355.e1-10. doi: 10.1038/onc.2012.240. Epub 2012 Jul 9. Oncogene. 2013. PMID: 22777356 Free PMC article. - MEK inhibition leads to PI3K/AKT activation by relieving a negative feedback on ERBB receptors.
Turke AB, Song Y, Costa C, Cook R, Arteaga CL, Asara JM, Engelman JA. Turke AB, et al. Cancer Res. 2012 Jul 1;72(13):3228-37. doi: 10.1158/0008-5472.CAN-11-3747. Epub 2012 May 2. Cancer Res. 2012. PMID: 22552284 Free PMC article. - Therapeutic target biomarkers of patient-derived xenograft models of gastric-type cervical adenocarcinoma.
Kojima Y, Yoshida H, Okuya T, Okuma HS, Nishikawa T, Tanioka M, Sudo K, Noguchi E, Shimoi T, Tamura K, Tanase Y, Uno M, Ishikawa M, Arakaki M, Ichikawa H, Yagishita S, Hamada A, Fujiwara Y, Yonemori K, Kato T. Kojima Y, et al. Gynecol Oncol Rep. 2023 Nov 14;50:101302. doi: 10.1016/j.gore.2023.101302. eCollection 2023 Dec. Gynecol Oncol Rep. 2023. PMID: 38054200 Free PMC article. - Pertuzumab, a novel HER dimerization inhibitor, inhibits the growth of human lung cancer cells mediated by the HER3 signaling pathway.
Sakai K, Yokote H, Murakami-Murofushi K, Tamura T, Saijo N, Nishio K. Sakai K, et al. Cancer Sci. 2007 Sep;98(9):1498-503. doi: 10.1111/j.1349-7006.2007.00553.x. Epub 2007 Jul 11. Cancer Sci. 2007. PMID: 17627612 Free PMC article.
References
- Breathnach, O. S., Freidlin, B., Conley, B., Green, M. R., Johnson, D. H., Gandara, D. R., O'Connell, M., Shepherd, F. A. & Johnson, B. E. (2001) J. Clin. Oncol. 19, 1734-1742. - PubMed
- Schiller, J. H., Harrington, D., Belani, C. P., Langer, C., Sandler, A., Krook, J., Zhu, J. & Johnson, D. H. (2002) N. Engl. J. Med. 346, 92-98. - PubMed
- Laskin, J. J. & Sandler, A. B. (2004) Cancer Treat. Rev. 30, 1-17. - PubMed
- Riese, D. J., II, & Stern, D. F. (1998) Bioessays 20, 41-48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 CA090578/CA/NCI NIH HHS/United States
- 5P01 CA 089021/CA/NCI NIH HHS/United States
- P20 CA 90578-02/CA/NCI NIH HHS/United States
- T32 CA009172/CA/NCI NIH HHS/United States
- R37 GM041890/GM/NIGMS NIH HHS/United States
- R01 GM041890/GM/NIGMS NIH HHS/United States
- 1K12 CA 87723-01/CA/NCI NIH HHS/United States
- K12 CA087723/CA/NCI NIH HHS/United States
- P01 CA089021/CA/NCI NIH HHS/United States
- 5T32 CA 09172-30/CA/NCI NIH HHS/United States
- GM 41890/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous