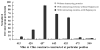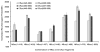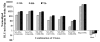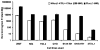Firefly luciferase enzyme fragment complementation for imaging in cells and living animals - PubMed (original) (raw)
Firefly luciferase enzyme fragment complementation for imaging in cells and living animals
Ramasamy Paulmurugan et al. Anal Chem. 2005.
Abstract
We identified different fragments of the firefly luciferase gene based on the crystal structure of firefly luciferase. These split reporter genes which encode for protein fragments, unlike the fragments currently used for studying protein-protein interactions, can self-complement and provide luciferase enzyme activity in different cell lines in culture and in living mice. The comparison of the fragment complementation associated recovery of firefly luciferase enzyme activity with intact firefly luciferase was estimated for different fragment combinations and ranged from 0.01 to 4% of the full firefly luciferase activity. Using a cooled optical charge-coupled device camera, the analysis of firefly luciferase fragment complementation in transiently transfected subcutaneous 293T cell implants in living mice showed significant detectable enzyme activity upon injecting d-luciferin, especially from the combinations of fragments identified (Nfluc and Cfluc are the N and C fragments of the firefly luciferase gene, respectively): Nfluc (1-475)/Cfluc (245-550), Nfluc (1-475)/Cfluc (265-550), and Nfluc (1-475)/Cfluc (300-550). The Cfluc (265-550) fragment, upon expression with the nuclear localization signal (NLS) peptide of SV40, shows reduced enzyme activity when the cells are cotransfected with the Nfluc (1-475) fragment expressed without NLS. We also proved in this study that the complementing fragments could be efficiently used for screening macromolecule delivery vehicles by delivering TAT-Cfluc (265-550) to cells stably expressing Nfluc (1-475) and recovering signal. These complementing fragments should be useful for many reporter-based assays including intracellular localization of proteins, studying cellular macromolecule delivery vehicles, studying cell-cell fusions, and also developing intracellular phosphorylation sensors based on fragment complementation.
Figures
Figure 1
(a) Schematic diagram showing the three-dimensional structure of firefly luciferase enzyme with indicated sites used for generating different NH2 and COOH (white) and only COOH (yellow) terminal fragments for the fragment complementation strategy. (b) Schematic diagram showing different vector constructs generated for the fragment complementation study for overlapping and nonoverlapping luciferase enzyme fragments with and without interacting proteins (FRB/FKBP12). The split sites are indicated after the amino acid positions.
Figure 2
Luminometer results of different nonoverlapping fragments with and without interacting proteins (FRB/FKBP12) studied in transiently transfected 293T cells and assayed 24 h posttransfection. The results are normalized by cotransfection of renilla luciferase. The result shows no significant fragment complementation from different nonoverlapping Nfluc and Cfluc fragments (fragments generated at split sites next to amino acid positions 415, 420, 437, 445, 455, 475, and 500). Four among the seven combinations showed significant signal upon exposure to rapamycin that brings Nfluc and Cfluc protein fragments together by interacting FRB and FKBP12 (split fragments generated at sites 420, 437, 445, and 455). The error bar is the standard error of the mean for three samples.
Figure 3
Luminometer assay conducted for different fragments generated for the fragment complementation assay using 293T cells. The results are normalized for transfection efficiency using cotransfection of renilla luciferase. The result shows different levels of fragment complementation associated luciferase activity resulting from different combinations of Nfluc and Cfluc fragments. The maximum level of activity was achieved from the cells cotransfected with Nfluc (1–475) and Cfluc (265–550). The luciferase signals from cells cotransfected with the combination of Nfluc and Cfluc without overlapping sequences were not significantly different from mock-transfected cells. At the same time, many combinations of overlapping fragments showed enzyme activity that was significantly (p < 0.05) above mock-transfected cells upon cotransfection. The error bar is the standard error of the mean for three samples.
Figure 4
Luminometer assay conducted for the 293T cells cotransfected with different combinations of Nfluc and Cfluc fragments studied at different time points (24, 48, and 72 h). The results are normalized using cotransfection of renilla luciferase. The result shows significant (p < 0.05) complementation-assisted firefly luciferase enzyme activity from different Nfluc fragments with three Cfluc fragments (245–550, 265–550, and 300–550) at all three time points studied. There is a slight increase in the activity from 24 to 48 h (significant p < 0.05) time point and no significant increase at 72 h. The error bar is the standard error of the mean for three samples.
Figure 5
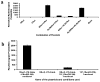
Luminometer assay conducted for the combination of Nfluc (1–475) and Cfluc (265–550) as compared to fully intact fluc (1–550) in 293T, N2a, HeLa, CHO, SK-N-SH, SH-SY-5Y, and 3T3-L1 cells. The result shows significant (p < 0.05) fragment complementation firefly luciferase enzyme activity in all cell lines as compared to mock-transfected cells. The error bar is the standard error of the mean for three samples.
Figure 6
In vivo imaging, using the optical CCD camera, conducted in mice with implants of 5 million cells cotransfected with Nfluc (1–475) and different Cfluc fragments in six different places: A (245–550), B (265–550), C (300–550), D (437–550), E (445–550), and F (500–550). The results show significant signal from the site implanted with the cotransfected Nfluc (1–475) with Cfluc (245–550), (265–550), and (300–550) in all time points studied. The other two combinations, Nfluc (1–475)/Cfluc (437–550) and Nfluc (1–475)/ Cfluc (445–550), showed minimum signals at the two higher time points studied. The cells cotransfected with the combinations of Nfluc (1–475) and Cfluc (500–550) showed no detectable signal at any of the time points studied.
Figure 7
(a) Luminometer assay conducted for the firefly luciferase enzyme fragment complementation by compartmentalizing the fragments in the same cellular localization. The cells cotransfected with either both the fragments without NLS or with NLS showed significant signal (N + C). The cells cotransfected with the combination fragments containing one fragment with NLS and the other without NLS showed signal that was significantly less (p < 0.01) than the previous one (N + NLS – C and NLS – N + C). The error bar is the standard error of the mean for three samples. (b) Fragment complementation mediated luciferase activity of 293T stable cells expressing Nfluc (1–475) with the transfection of Cfluc (265–550) and with the transduction of TAT–Cfluc (265–550). The results show significant complemented luciferase enzyme signal from the cells transfected with Cfluc (265–550) and transduced with TAT–Cfluc protein. The cells expressing only Nfluc (1–475) or Cfluc (265–550) show no significant luciferase enzyme signal. The error bar is the standard error of the mean for three samples.
Similar articles
- Combinatorial library screening for developing an improved split-firefly luciferase fragment-assisted complementation system for studying protein-protein interactions.
Paulmurugan R, Gambhir SS. Paulmurugan R, et al. Anal Chem. 2007 Mar 15;79(6):2346-53. doi: 10.1021/ac062053q. Epub 2007 Feb 13. Anal Chem. 2007. PMID: 17295448 Free PMC article. - Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals.
Luker KE, Smith MC, Luker GD, Gammon ST, Piwnica-Worms H, Piwnica-Worms D. Luker KE, et al. Proc Natl Acad Sci U S A. 2004 Aug 17;101(33):12288-93. doi: 10.1073/pnas.0404041101. Epub 2004 Jul 29. Proc Natl Acad Sci U S A. 2004. PMID: 15284440 Free PMC article. - Id protein-firefly luciferase N-fragment & firefly luciferase C-fragment-MyoD protein.
Zhang H. Zhang H. 2008 Sep 24 [updated 2008 Oct 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Sep 24 [updated 2008 Oct 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641378 Free Books & Documents. Review. - Construction and validation of improved triple fusion reporter gene vectors for molecular imaging of living subjects.
Ray P, Tsien R, Gambhir SS. Ray P, et al. Cancer Res. 2007 Apr 1;67(7):3085-93. doi: 10.1158/0008-5472.CAN-06-2402. Cancer Res. 2007. PMID: 17409415 - NFluc-FHA2-Aktpep-CFluc.
Zhang H. Zhang H. 2008 Nov 14 [updated 2008 Dec 22]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Nov 14 [updated 2008 Dec 22]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641681 Free Books & Documents. Review.
Cited by
- Human GH receptor-IGF-1 receptor interaction: implications for GH signaling.
Gan Y, Buckels A, Liu Y, Zhang Y, Paterson AJ, Jiang J, Zinn KR, Frank SJ. Gan Y, et al. Mol Endocrinol. 2014 Nov;28(11):1841-54. doi: 10.1210/me.2014-1174. Epub 2014 Sep 11. Mol Endocrinol. 2014. PMID: 25211187 Free PMC article. - Bright Molecular Strain Probe Templates for Reporting Protein-Protein Interactions.
Kim SB, Furuta T, Kamiya G, Kitada N, Paulmurugan R, Maki SA. Kim SB, et al. Sensors (Basel). 2023 Mar 27;23(7):3498. doi: 10.3390/s23073498. Sensors (Basel). 2023. PMID: 37050557 Free PMC article. - Intelligent design of nano-scale molecular imaging agents.
Kim SB, Hattori M, Ozawa T. Kim SB, et al. Int J Mol Sci. 2012 Dec 12;13(12):16986-7005. doi: 10.3390/ijms131216986. Int J Mol Sci. 2012. PMID: 23235326 Free PMC article. Review. - Biophysical Techniques for Target Validation and Drug Discovery in Transcription-Targeted Therapy.
Moustaqil M, Gambin Y, Sierecki E. Moustaqil M, et al. Int J Mol Sci. 2020 Mar 26;21(7):2301. doi: 10.3390/ijms21072301. Int J Mol Sci. 2020. PMID: 32225120 Free PMC article. Review. - Supramolecular Control over Split-Luciferase Complementation.
Bosmans RP, Briels JM, Milroy LG, de Greef TF, Merkx M, Brunsveld L. Bosmans RP, et al. Angew Chem Int Ed Engl. 2016 Jul 25;55(31):8899-903. doi: 10.1002/anie.201602807. Epub 2016 Jun 29. Angew Chem Int Ed Engl. 2016. PMID: 27356091 Free PMC article.
References
- Fujikawa H, Morozumi S. Shokuhin Eiseigaku Zasshi. 2003;44:83–88. - PubMed
- Olsson T, Sandstedt K, Holmberg O, Thore A. Biotechnol Appl Biochem. 1986;8:361–369. - PubMed
- Verhees J, van der Kro lA, Vreugdenhil D, van der Plas L. Plant Mol Biol. 2002;50:653–665. - PubMed
- Contag CH, Spilman SD, Contag PR, Oshiro M, Eames B, Dennery P, Stevenson DK, Benaron DA. Photochem Photobiol. 1997;66:523–531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous