Inactivation of liver X receptor beta leads to adult-onset motor neuron degeneration in male mice - PubMed (original) (raw)
Inactivation of liver X receptor beta leads to adult-onset motor neuron degeneration in male mice
Sandra Andersson et al. Proc Natl Acad Sci U S A. 2005.
Erratum in
- Proc Natl Acad Sci U S A. 2006 May 23;103(21):8298
Abstract
Male mice with inactivated liver X receptor (LXR) beta suffer from adult-onset motor neuron degeneration. By 7 months of age, motor coordination is impaired, and this condition is associated with lipid accumulation and loss of motor neurons in the spinal cord, together with axonal atrophy and astrogliosis. Several of these features are reminiscent of the neuropathological signs of chronic motor neuron disease such as amyotrophic lateral sclerosis. Because the LXRs are important for cholesterol and lipid metabolism, we speculate that absence of LXRbeta leads to pathological accumulation of sterols and lipids that may themselves be neurotoxic or may modulate intracellular pathways and thereby predispose motor neurons to degeneration.
Figures
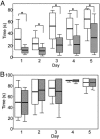
Fig. 1.
Box plot of rota-rod performance in 7-month-old mice. Five trials per day were given for 5 consecutive days. The retention time of mice on the rod was recorded, and mice staying for 90 sec were taken from the rod and recorded as 90 sec. White bars represent WT and striped bars represent LXRβ-/- (n = 10-13 for each group; box: mean ± SD; whisker: min, max; *, P < 0.01, Student's t test). (A) The LXRβ-/- male mice performed better for each day as the trial went on and they learned equally as well as the WT mice, but for the male LXRβ-/- mice, the retention time to stay on the rod was significantly shorter than for WT mice for all 5 days. (B) Female LXRβ-/- performed as well as the WT females, and both the learning ability and retention time to stay on the rod for all of the 5 days were normal.
Fig. 2.
Neuromuscular junction immunohistochemistry. (A and D) Motor endplates were identified with tetramethylrhodamine conjugated α-bungarotoxin (red). (B and E) Synapsin I, seen in green, was used as a marker for axon innervation. (C) Innervated endplate with yellow, indicating overlap of motor endplates with synapsin I staining. (F) Denervated endplates with no overlap of synapsin I staining. (Bar, 10 μm.)
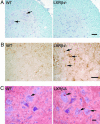
Fig. 3.
Neuropathology in the lateroventral horns of L1 segments of the spinal cord in 7-month-old male mice. (A) Luxol fast blue was used to stain myelin, and counterstaining with Mayer's hematoxylin was performed to visualize cell nuclei. No evidence of demyelination was found in LXRβ-/- as compared with WT mice. However, loss of large α-motor neurons can be seen in the LXRβ-/- mice. Arrows indicate α-motor neurons in WT mice. The number of motor neurons was evaluated by counting large ChAT-positive cells and this count gave 6 ± 1 motor neurons per section for WT and 4 ± 1 motor neurons per section for LXRβ-/- mice, P < 0.001; Student's t test; n = 8 per genotype. Note that the picture only shows one of the two ventral horns in a section. (Bar, 50 μm.) (B) GFAP staining of astroglia can be seen as brown star-like shaped cell bodies, as indicated with arrows in LXRβ-/- sample. More activated astrocytes with large cell bodies can be seen in LXRβ-/- mice as compared with WT. Counting the number of GFAP-positive cells gave 12 ± 2 astrocytes per section for WT and 16 ± 3 astrocytes per section for LXRβ-/- mice; P = 0.004; Student's t test; n = 8 per genotype. Note that the picture only shows one of the two ventral horns in a section. (Bar, 50 μm.) (C) Lipid deposits were revealed by staining with Oil red O, as indicated by arrows. More and lager deposits can be seen in motor neurons of LXRβ-/- mice compared with WT mice; n = 5 per genotype. (Bar, 20 μm.)
Fig. 4.
Toluidine blue staining of L1 ventral nerve roots. The size of the axon calibers is smaller in LXRβ-/- mice as compared with WT, perhaps indicating axonal atrophy. Measurement of the mean diameter gave 4.1 ± 0.3 μm for WT and 3.7 ± 0.1 μm for LXRβ-/- mice; P = 0.022; Student's t test; n = 5 per genotype. (Insets) Higher magnification of selected parts can be seen. (Bar, 50 μm.)
Fig. 5.
Western blot of cerebellar extract from 7-month-old male mice. No difference in protein expression of either of the two calcium-buffering proteins, calretinin or calbindin, could be detected in LXRβ-/- mice as compared with WT. Size ladder is indicated on the left. α-tubulin (66 kDa) was used as a loading control (n = 3 for each genotype). (A) The protein expression of calretinin (≈30 kDa), mainly produced by granule cells in the cerebellum, did not differ in LXRβ-/- mice compared with WT. (B) The protein expression of calbindin (28 kDa), mainly produced by Purkinje cells in the cerebellum, did not differ in LXRβ-/- mice compared with WT.
Similar articles
- Neuropathologic and biochemical changes during disease progression in liver X receptor beta-/- mice, a model of adult neuron disease.
Bigini P, Steffensen KR, Ferrario A, Diomede L, Ferrara G, Barbera S, Salzano S, Fumagalli E, Ghezzi P, Mennini T, Gustafsson JA. Bigini P, et al. J Neuropathol Exp Neurol. 2010 Jun;69(6):593-605. doi: 10.1097/NEN.0b013e3181df20e1. J Neuropathol Exp Neurol. 2010. PMID: 20467332 - Liver X receptor beta (LXRbeta): a link between beta-sitosterol and amyotrophic lateral sclerosis-Parkinson's dementia.
Kim HJ, Fan X, Gabbi C, Yakimchuk K, Parini P, Warner M, Gustafsson JA. Kim HJ, et al. Proc Natl Acad Sci U S A. 2008 Feb 12;105(6):2094-9. doi: 10.1073/pnas.0711599105. Epub 2008 Jan 31. Proc Natl Acad Sci U S A. 2008. PMID: 18238900 Free PMC article. - Liver X receptors in the central nervous system: from lipid homeostasis to neuronal degeneration.
Wang L, Schuster GU, Hultenby K, Zhang Q, Andersson S, Gustafsson JA. Wang L, et al. Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13878-83. doi: 10.1073/pnas.172510899. Epub 2002 Oct 4. Proc Natl Acad Sci U S A. 2002. PMID: 12368482 Free PMC article. - Liver X receptors and atherosclerosis.
Lala DS. Lala DS. IDrugs. 2004 Jun;7(6):563-9. IDrugs. 2004. PMID: 15197661 Review. - Liver X receptors as integrators of metabolic and inflammatory signaling.
Zelcer N, Tontonoz P. Zelcer N, et al. J Clin Invest. 2006 Mar;116(3):607-14. doi: 10.1172/JCI27883. J Clin Invest. 2006. PMID: 16511593 Free PMC article. Review.
Cited by
- Liver X receptors and cholesterol metabolism: role in ventral midbrain development and neurodegeneration.
Theofilopoulos S, Arenas E. Theofilopoulos S, et al. F1000Prime Rep. 2015 Apr 2;7:37. doi: 10.12703/P7-37. eCollection 2015. F1000Prime Rep. 2015. PMID: 26097711 Free PMC article. Review. - Anxiety in liver X receptor β knockout female mice with loss of glutamic acid decarboxylase in ventromedial prefrontal cortex.
Tan XJ, Dai YB, Wu WF, Warner M, Gustafsson JÅ. Tan XJ, et al. Proc Natl Acad Sci U S A. 2012 May 8;109(19):7493-8. doi: 10.1073/pnas.1205189109. Epub 2012 Apr 23. Proc Natl Acad Sci U S A. 2012. PMID: 22529354 Free PMC article. - Liver X receptors: from cholesterol regulation to neuroprotection-a new barrier against neurodegeneration in amyotrophic lateral sclerosis?
Mouzat K, Raoul C, Polge A, Kantar J, Camu W, Lumbroso S. Mouzat K, et al. Cell Mol Life Sci. 2016 Oct;73(20):3801-8. doi: 10.1007/s00018-016-2330-y. Epub 2016 Aug 10. Cell Mol Life Sci. 2016. PMID: 27510420 Free PMC article. Review. - Nuclear receptors in neurodegenerative diseases.
Skerrett R, Malm T, Landreth G. Skerrett R, et al. Neurobiol Dis. 2014 Dec;72 Pt A:104-16. doi: 10.1016/j.nbd.2014.05.019. Epub 2014 May 27. Neurobiol Dis. 2014. PMID: 24874548 Free PMC article. Review. - Cholesterolomics: An update.
Griffiths WJ, Abdel-Khalik J, Yutuc E, Morgan AH, Gilmore I, Hearn T, Wang Y. Griffiths WJ, et al. Anal Biochem. 2017 May 1;524:56-67. doi: 10.1016/j.ab.2017.01.009. Epub 2017 Jan 10. Anal Biochem. 2017. PMID: 28087213 Free PMC article. Review.
References
- Alberti, S., Steffensen, K. R. & Gustafsson, J. A. (2000) Gene 243, 93-103. - PubMed
- Janowski, B. A., Willy, P. J., Devi, T. R., Falck, J. R. & Mangelsdorf, D. J. (1996) Nature 383, 728-731. - PubMed
- Lehmann, J. M., Kliewer, S. A., Moore, L. B., Smith-Oliver, T. A., Oliver, B. B., Su, J. L., Sundseth, S. S., Winegar, D. A., Blanchard, D. E., Spencer, T. A. & Willson, T. M. (1997) J. Biol. Chem. 272, 3137-3140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases