Octamer and Sox elements are required for transcriptional cis regulation of Nanog gene expression - PubMed (original) (raw)
Octamer and Sox elements are required for transcriptional cis regulation of Nanog gene expression
Takao Kuroda et al. Mol Cell Biol. 2005 Mar.
Abstract
The pluripotential cell-specific gene Nanog encodes a homeodomain-bearing transcription factor required for maintaining the undifferentiated state of stem cells. However, the molecular mechanisms that regulate Nanog gene expression are largely unknown. To address this important issue, we used luciferase assays to monitor the relative activities of deletion fragments from the 5'-flanking region of the gene. An adjacent pair of highly conserved Octamer- and Sox-binding sites was found to be essential for activating pluripotential state-specific gene expression. Furthermore, the 5'-end fragment encompassing the Octamer/Sox element was sufficient for inducing the proper expression of a green fluorescent protein reporter gene even in human embryonic stem (ES) cells. The potential of OCT4 and SOX2 to bind to this element was verified by electrophoretic mobility shift assays with extracts from F9 embryonal carcinoma cells and embryonic germ cells derived from embryonic day 12.5 embryos. However, in ES cell extracts, a complex of OCT4 with an undefined factor preferentially bound to the Octamer/Sox element. Thus, Nanog transcription may be regulated through an interaction between Oct4 and Sox2 or a novel pluripotential cell-specific Sox element-binding factor which is prominent in ES cells.
Figures
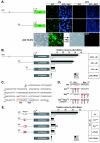
FIG. 1.
Undifferentiated state-specific expression of the LR/_Nanog_-GFP transgene. (A) Structure of the LR/_Nanog_-GFP transgene containing 5′- and 3′-flanking regions. P, PvuII; X, XbaI; B, BglII; N, NcoI. (B) Southern blot hybridization analysis of TG6 and TG7 transgenic cell lines and the parental R1 ES cell line. The transgene-specific 3.4-kb PvuII-BglII fragment and the 4.1-kb XbaI-NcoI fragment were detected with 5′ and 3′ probes, respectively. (C) Expression of GFP in undifferentiated (UD) TG6 ES cells. GFP expression was visualized by fluorescence microscopy and Western blot hybridization analysis with anti-GFP antibody. Histone H3 was used as a control. (D) Expression of GFP restricted to undifferentiated ES cells located in the middle of 5-day-old EBs and in the center of colonies 3 days after culturing of 5-day-old EBs. (E) Down-regulation of GFP expression by in vitro differentiation with retinoic acid (RA) treatment for 5 days. (F) Western blot hybridization analysis of GFP and endogenous NANOG during RA-induced cell differentiation. Histone H3 was used as a control.
FIG. 2.
Octamer and Sox elements are required for Nanog expression. (A) Transient expression of GFP transgenes with −2342 or −332 5′-end fragments in R1 ES and NIH 3T3 cells. Transcriptional down-regulation of GFP was detected in differentiated −332-GFP TG ES (−332 TG ES) cells by treatment with retinoic acid (RA). (B) Luciferase assays with deletion constructs in R1 ES, F9 EC, and NIH 3T3 cells. Luciferase activities are shown relative to those of pGL3-Basic. Bars represent the means ± standard errors of three independent experiments. (C) DNA sequence of the mouse 5′-flanking region between positions −332 and −154. Octamer (Oct) and Sox elements are outlined in red and blue, respectively. (D) Sequence mutations introduced into Octamer and/or Sox elements. (E) Luciferase assays with the −332 5′-end fragment with or without mutations in Octamer and/or Sox elements in R1 ES, F9 EC, and NIH 3T3 cells. Luciferase activities are shown relative to those of pGL3-Basic. Bars represent the means ± standard errors of three independent experiments.
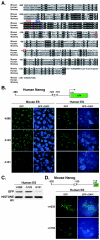
FIG. 3.
Expression of the human Nanog reporter gene in mouse and human ES cells. (A) Comparative DNA sequence analysis of the Nanog 5′-flanking regions of mice, monkeys, and humans. Octamer (Oct) and Sox elements are outlined in red and blue, respectively. Red circles show putative transcriptional start sites. Identical nucleotides are highlighted in black. (B) Transient GFP expression under the regulation of the human Nanog promoter in mouse and human ES cells. The −h380 and −h123 5′-end fragments contain both Octamerand Sox elements, while the −h101 5′-end fragment does not. (C) Western blot hybridization analysis of GFP expression in human ES cells. Histone H3 was used as a control. (D) Transient GFP expression under the regulation of the mouse Nanog promoter in human ES cells. The −m332 5′-end fragment contains the Octamer and Sox elements, while the −m153 5′-end fragment does not.
FIG. 4.
Binding of exogenous OCT4 and SOX2 to Octamer and Sox elements in COS-1 cells. (A) Western blot hybridization analysis of exogenous Myc-tagged OCT4 and HA-tagged SOX2 expression with anti-OCT4, anti-SOX2, anti-Myc, and anti-HA antibodies. Actin was used as a control. (B) DNA sequences of Nanog and Fgf4 probes. Octamer (Oct) and Sox elements are outlined. (C) EMSA with Nanog and Fgf4 probes and COS-1 cells. Bands of the OCT4-DNA, SOX2-DNA, and OCT4/SOX2-DNA complexes are indicated. (D) Cotransfection reporter assays with Oct4 and Sox2 expression constructs in NIH 3T3 cells. Bars represent the means ± standard errors of three independent experiments.
FIG. 5.
Binding of endogenous OCT4 and SOX2 to Octamer and Sox elements in F9 EC cells. (A) Western blot hybridization analysis of endogenous OCT4, SOX2, and NANOG in nuclear extracts (N.E.) and cytoplasmic extracts (C.E.) of F9 EC and R1 ES cells. (B) EMSA with the Nanog probe and nuclear extracts of COS-1 and F9 EC cells. Bands of the OCT4-DNA, SOX2-DNA, and OCT4/SOX2-DNA complexes are indicated. (C) Competition assays with unlabeled probes with or without mutations in Octamer and/or Sox elements. Relative amounts of binding proteins (B.P.) are indicated by grey bars. Bands of the OCT4-DNA and OCT4/SOX2-DNA complexes are indicated. Octmut (Om), Nanog probe with triple mutations in the Octamer element; Soxmut (Sm), Nanog probe with triple mutations in the Sox element. (D) Supershift assay with anti-OCT4 or anti-SOX2 antibody and F9 EC cell nuclear extracts. Bands of the OCT4-DNA and OCT4/SOX2-DNA complexes are indicated by arrows, and supershifted bands are indicated by asterisks. Rabbit IgG and goat IgG were used as controls. (E) Chromatin immunoprecipitation assay demonstrating the in vivo potential of OCT4 and SOX2 to bind to Nanog and Fgf4 Octamer and Sox elements, respectively.
FIG. 6.
Binding of endogenous OCT4 and PSBP to Octamer and Sox elements in R1 ES cells. Bands of the OCT4-DNA and OCT4/SOX2-DNA complexes are indicated by arrows, bands of the OCT4/PSBP-DNA complex are indicated by open circles, and supershifted bands are indicated by asterisks. (A) EMSA with Nanog and Fgf4 probes and nuclear extracts of F9 EC, TMA-58G EG, and R1 ES cells. (B) Competition assay with unlabeled probes with or without mutations in Octamer and/or Sox elements. (C) Supershift assay with anti-OCT4 or anti-SOX2 antibody and R1 ES cell nuclear extracts. Rabbit IgG and goat IgG were used as controls. (D) Schematic model for transcriptional regulation of Nanog in ES cells. In R1 ES cells but not in F9 EC cells, the OCT4/PSBP complex dominantly up-regulates Nanog transcription by binding to the Octamer/Sox element. PSBP binds to the Sox element with a greater affinity than SOX2.
Similar articles
- Transcriptional regulation of nanog by OCT4 and SOX2.
Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P. Rodda DJ, et al. J Biol Chem. 2005 Jul 1;280(26):24731-7. doi: 10.1074/jbc.M502573200. Epub 2005 Apr 27. J Biol Chem. 2005. PMID: 15860457 - Transcriptional regulation of oct4 in human bone marrow mesenchymal stem cells.
Wei X, Shen CY. Wei X, et al. Stem Cells Dev. 2011 Mar;20(3):441-9. doi: 10.1089/scd.2010.0069. Epub 2010 Sep 13. Stem Cells Dev. 2011. PMID: 20594032 - Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells.
Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS, Li P, Ang YS, Lim B, Robson P, Ng HH. Chew JL, et al. Mol Cell Biol. 2005 Jul;25(14):6031-46. doi: 10.1128/MCB.25.14.6031-6046.2005. Mol Cell Biol. 2005. PMID: 15988017 Free PMC article. - Self-renewal of teratocarcinoma and embryonic stem cells.
Chambers I, Smith A. Chambers I, et al. Oncogene. 2004 Sep 20;23(43):7150-60. doi: 10.1038/sj.onc.1207930. Oncogene. 2004. PMID: 15378075 Review. - Transcriptional regulatory networks in embryonic stem cells.
Chen X, Vega VB, Ng HH. Chen X, et al. Cold Spring Harb Symp Quant Biol. 2008;73:203-9. doi: 10.1101/sqb.2008.73.026. Epub 2008 Nov 6. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19022762 Review.
Cited by
- TFEB regulates pluripotency transcriptional network in mouse embryonic stem cells independent of autophagy-lysosomal biogenesis.
Tan A, Prasad R, Jho EH. Tan A, et al. Cell Death Dis. 2021 Apr 1;12(4):343. doi: 10.1038/s41419-021-03632-9. Cell Death Dis. 2021. PMID: 33795648 Free PMC article. - The Roles of the Stem Cell-Controlling Sox2 Transcription Factor: from Neuroectoderm Development to Alzheimer's Disease?
Sarlak G, Vincent B. Sarlak G, et al. Mol Neurobiol. 2016 Apr;53(3):1679-1698. doi: 10.1007/s12035-015-9123-4. Epub 2015 Feb 18. Mol Neurobiol. 2016. PMID: 25691455 Review. - The EWS-Oct-4 fusion gene encodes a transforming gene.
Lee J, Kim JY, Kang IY, Kim HK, Han YM, Kim J. Lee J, et al. Biochem J. 2007 Sep 15;406(3):519-26. doi: 10.1042/BJ20070243. Biochem J. 2007. PMID: 17564582 Free PMC article. - Effect of overexpression of Oct4 and Sox2 genes on the biological and oncological characteristics of gastric cancer cells.
Chen B, Zhu Z, Li L, Ye W, Zeng J, Gao J, Wang S, Zhang L, Huang Z. Chen B, et al. Onco Targets Ther. 2019 Jun 18;12:4667-4682. doi: 10.2147/OTT.S209734. eCollection 2019. Onco Targets Ther. 2019. PMID: 31417271 Free PMC article. - Stem cells and TCF proteins: a role for beta-catenin--independent functions.
Yi F, Merrill BJ. Yi F, et al. Stem Cell Rev. 2007 Jan;3(1):39-48. doi: 10.1007/s12015-007-0003-9. Stem Cell Rev. 2007. PMID: 17873380 Review.
References
- Ben-Shushan, E., J. R. Thompson, L. J. Gudas, and Y. Bergman. 1998. Rex-1, a gene encoding a transcription factor expressed in the early embryo, is regulated via Oct-3/4 and Oct-6 binding to an octamer site and a novel protein, Rox-1, binding to an adjacent site. Mol. Cell. Biol. 18:1866-1878. - PMC - PubMed
- Chambers, I., D. Colby, M. Robertson, J. Nichols, S. Lee, S. Tweedie, and A. Smith. 2003. Functional expression cloning of nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113:643-655. - PubMed
- Czermin, B., R. Melfi, D. McCabe, V. Seitz, A. Imhof, and V. Pirrotta. 2002. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111:185-196. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous