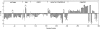Detection of lung cancer by sensor array analyses of exhaled breath - PubMed (original) (raw)
Clinical Trial
. 2005 Jun 1;171(11):1286-91.
doi: 10.1164/rccm.200409-1184OC. Epub 2005 Mar 4.
Daniel Laskowski, Olivia Deffenderfer, Timothy Burch, Shuo Zheng, Peter J Mazzone, Tarek Mekhail, Constance Jennings, James K Stoller, Jacqueline Pyle, Jennifer Duncan, Raed A Dweik, Serpil C Erzurum
Affiliations
- PMID: 15750044
- PMCID: PMC2718462
- DOI: 10.1164/rccm.200409-1184OC
Clinical Trial
Detection of lung cancer by sensor array analyses of exhaled breath
Roberto F Machado et al. Am J Respir Crit Care Med. 2005.
Abstract
Rationale: Electronic noses are successfully used in commercial applications, including detection and analysis of volatile organic compounds in the food industry.
Objectives: We hypothesized that the electronic nose could identify and discriminate between lung diseases, especially bronchogenic carcinoma.
Methods: In a discovery and training phase, exhaled breath of 14 individuals with bronchogenic carcinoma and 45 healthy control subjects or control subjects without cancer was analyzed. Principal components and canonic discriminant analysis of the sensor data was used to determine whether exhaled gases could discriminate between cancer and noncancer. Discrimination between classes was performed using Mahalanobis distance. Support vector machine analysis was used to create and apply a cancer prediction model prospectively in a separate group of 76 individuals, 14 with and 62 without cancer.
Main results: Principal components and canonic discriminant analysis demonstrated discrimination between samples from patients with lung cancer and those from other groups. In the validation study, the electronic nose had 71.4% sensitivity and 91.9% specificity for detecting lung cancer; positive and negative predictive values were 66.6 and 93.4%, respectively. In this population with a lung cancer prevalence of 18%, positive and negative predictive values were 66.6 and 94.5%, respectively.
Conclusion: The exhaled breath of patients with lung cancer has distinct characteristics that can be identified with an electronic nose. The results provide feasibility to the concept of using the electronic nose for managing and detecting lung cancer.
Figures
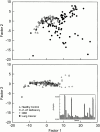
**Figure 1.
Principal components analysis plot, shown as a two-dimensional projection of the 32-dimensional vector analyses, demonstrates distinct clustering of the samples from patients with lung cancer separate from healthy control subjects (upper panel), whereas patients with interstitial lung disease or emphysema are not separable from healthy control subjects (lower panel). Inset: Example of a typical smellprint derived from the 32 sensor responses, which are used in the multidimensional analyses, from a healthy control subject (black bars) and a patient with lung cancer (gray bars). α1-AT = α1-antitrypsin; CBD = chronic pulmonary beryllium disease; R = postmeasurement sensor resistance; Ro = baseline sensor resistance.
**Figure 2.
Support vector machine (SVM) classification for lung cancer. During classification, SVM calculates the distance of the unknown sample from the decision boundary in the model it has learned. In this graph, the margin for each breath sample is shown, with a positive value indicating classification of lung cancer (i.e., how far within the lung cancer boundary the sample falls). A minimum of five analyses performed on each individual's exhaled breath is shown. A negative value indicates a noncancer classification, with the value indicating how far outside of the lung cancer boundary the sample falls. The incorrect classification of a sample is identified by the open circles at the end of the line, and correct classification by closed circles. The majority of predictions were concordant (i.e., all five classifications of an individual the same in 92% of cases). Discordance occurred in 8% of cases. Assignment as cancer was predicted based on the predominant response (three or more of five) for that particular patient. Incorrect classification of lung cancer as noncancer is noted for two individuals with small cell carcinoma (f, g: all five analyses for each predict noncancer) and two individuals with relatively small primary lesions (h: four of five analyses predict noncancer; i: all five analyses for each predict noncancer). Incorrect classification of control subjects as cancer is noted for an individual with asthma with severe airflow limitation (a: three of five analyses cancer prediction), an individual with primary pulmonary hypertension (PAH; b: all five analyses predict cancer), and three healthy nonsmoking control subjects with no known lung disease (c, e: four analyses predict cancer; d: all five analyses predict cancer). *Indicates breath samples from two different individuals with lung cancer after curative resection of cancer.
Comment in
- Can the electronic nose really sniff out lung cancer?
Phillips M. Phillips M. Am J Respir Crit Care Med. 2005 Oct 15;172(8):1060; author reply 1060-1. doi: 10.1164/ajrccm.172.8.958. Am J Respir Crit Care Med. 2005. PMID: 16216842 No abstract available.
Similar articles
- Volatile signature for the early diagnosis of lung cancer.
Gasparri R, Santonico M, Valentini C, Sedda G, Borri A, Petrella F, Maisonneuve P, Pennazza G, D'Amico A, Di Natale C, Paolesse R, Spaggiari L. Gasparri R, et al. J Breath Res. 2016 Feb 9;10(1):016007. doi: 10.1088/1752-7155/10/1/016007. J Breath Res. 2016. PMID: 26857451 - Integration of electronic nose technology with spirometry: validation of a new approach for exhaled breath analysis.
de Vries R, Brinkman P, van der Schee MP, Fens N, Dijkers E, Bootsma SK, de Jongh FH, Sterk PJ. de Vries R, et al. J Breath Res. 2015 Oct 15;9(4):046001. doi: 10.1088/1752-7155/9/4/046001. J Breath Res. 2015. PMID: 26469298 - Sex and Smoking Status Effects on the Early Detection of Early Lung Cancer in High-Risk Smokers Using an Electronic Nose.
McWilliams A, Beigi P, Srinidhi A, Lam S, MacAulay CE. McWilliams A, et al. IEEE Trans Biomed Eng. 2015 Aug;62(8):2044-54. doi: 10.1109/TBME.2015.2409092. Epub 2015 Mar 11. IEEE Trans Biomed Eng. 2015. PMID: 25775482 - Electronic Nose Analysis of Exhaled Breath Volatiles to Identify Lung Cancer Cases: A Systematic Review.
Swanson B, Fogg L, Julion W, Arrieta MT. Swanson B, et al. J Assoc Nurses AIDS Care. 2020 Jan-Feb;31(1):71-79. doi: 10.1097/JNC.0000000000000146. J Assoc Nurses AIDS Care. 2020. PMID: 31860595 Review. - Electronic Nose Technology in Respiratory Diseases.
Dragonieri S, Pennazza G, Carratu P, Resta O. Dragonieri S, et al. Lung. 2017 Apr;195(2):157-165. doi: 10.1007/s00408-017-9987-3. Epub 2017 Feb 25. Lung. 2017. PMID: 28238110 Review.
Cited by
- The human volatilome meets cancer diagnostics: past, present, and future of noninvasive applications.
Barbosa JMG, Filho NRA. Barbosa JMG, et al. Metabolomics. 2024 Oct 7;20(5):113. doi: 10.1007/s11306-024-02180-5. Metabolomics. 2024. PMID: 39375265 Review. - Electronic Nose Analysis of Exhaled Breath Volatile Organic Compound Profiles during Normoxia, Hypoxia, and Hyperoxia.
Tondo P, Scioscia G, Di Marco M, Quaranta VN, Campanino T, Palmieri G, Portacci A, Santamato A, Lacedonia D, Carpagnano GE, Dragonieri S. Tondo P, et al. Molecules. 2024 Sep 13;29(18):4358. doi: 10.3390/molecules29184358. Molecules. 2024. PMID: 39339353 Free PMC article. - Electronic Noses: From Gas-Sensitive Components and Practical Applications to Data Processing.
Zhai Z, Liu Y, Li C, Wang D, Wu H. Zhai Z, et al. Sensors (Basel). 2024 Jul 24;24(15):4806. doi: 10.3390/s24154806. Sensors (Basel). 2024. PMID: 39123852 Free PMC article. Review. - Cross-site validation of lung cancer diagnosis by electronic nose with deep learning: a multicenter prospective study.
Lee MR, Kao MH, Hsieh YC, Sun M, Tang KT, Wang JY, Ho CC, Shih JY, Yu CJ. Lee MR, et al. Respir Res. 2024 May 10;25(1):203. doi: 10.1186/s12931-024-02840-z. Respir Res. 2024. PMID: 38730430 Free PMC article. - Breath Analysis for Lung Cancer Early Detection-A Clinical Study.
Jia Z, Thavasi V, Venkatesan T, Lee P. Jia Z, et al. Metabolites. 2023 Dec 12;13(12):1197. doi: 10.3390/metabo13121197. Metabolites. 2023. PMID: 38132879 Free PMC article.
References
- Munoz BC, Steinthal G, Sunshine S. Conductive polymer-carbon black composites-based sensor arrays for use in an electronic nose. Sensor Rev 1999;19:300–305.
- Kermany BG, Schiffman SS, Nagle HT. A novel method for reducing the dimensionality in a sensor array. IEEE Trans Instr Meas 1998;47:728–741.
- Kermany BG, Schiffman SS, Nagle HT. Using neural networks and genetic algorithms to enhance performance in an electronic nose. IEEE Trans Biomed Eng 1999;46:429–439. - PubMed
- Gardner JW, Bartlett PN. Electronic noses: principles and applications. Oxford, UK/New York: Oxford University Press; 1999.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical