Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons - PubMed (original) (raw)
Comparative Study
doi: 10.1038/nn1419. Epub 2005 Mar 6.
Affiliations
- PMID: 15750589
- PMCID: PMC1628303
- DOI: 10.1038/nn1419
Comparative Study
Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons
Sara J Aton et al. Nat Neurosci. 2005 Apr.
Abstract
The mammalian suprachiasmatic nucleus (SCN) is a master circadian pacemaker. It is not known which SCN neurons are autonomous pacemakers or how they synchronize their daily firing rhythms to coordinate circadian behavior. Vasoactive intestinal polypeptide (VIP) and the VIP receptor VPAC(2) (encoded by the gene Vipr2) may mediate rhythms in individual SCN neurons, synchrony between neurons, or both. We found that Vip(-/-) and Vipr2(-/-) mice showed two daily bouts of activity in a skeleton photoperiod and multiple circadian periods in constant darkness. Loss of VIP or VPAC(2) also abolished circadian firing rhythms in approximately half of all SCN neurons and disrupted synchrony between rhythmic neurons. Critically, daily application of a VPAC(2) agonist restored rhythmicity and synchrony to VIP(-/-) SCN neurons, but not to Vipr2(-/-) neurons. We conclude that VIP coordinates daily rhythms in the SCN and behavior by synchronizing a small population of pacemaking neurons and maintaining rhythmicity in a larger subset of neurons.
Figures
Figure 1
Mice with disrupted VIP/VPAC2 signaling express multiple circadian periods. (a,c) Double-plotted actograms showing representative wheel-running activity of wild-type (C57Bl/6), _Vip_−/− and _Vipr2_−/− mice in a light/dark schedule (days 1–10), a skeleton photoperiod (days 11–20), and constant darkness (days 20–90). Gray shading indicates the time when lights were on. VIP and VPAC2 mutant mice appeared normal while on a 12 h/12 h light/dark schedule but were active during both dark phases each day in a skeleton photoperiod, whereas wild-type mice were strictly nocturnal. (b,d) Peaks in the corresponding χ2 periodogram for each animal show the dominant and secondary period(s) during days 21–90 in constant darkness. Peaks above the diagonal line (indicating the 99.9% confidence level) between 16 and 34 h were considered significant circadian periods. Note that mutants in a and b (representing two-thirds of mutants of both genotypes) expressed multiple circadian periods, whereas those in c and d (representing one-third of mutants) showed more coherent circadian behavior, with a single, shorter, free-running period. (e) Mice of all three genotypes expressed significant circadian periodicity. The percentage of mice expressing a single circadian period (gray) was higher in wild-type mice than in the two mutant genotypes, whereas a large percentage of mutant mice expressed multiple circadian periods (black). (f) The circadian amplitude of rhythms was diminished in both mutants relative to wild-type. Bars show circadian amplitudes (mean ± s.e.m.) at the dominant period as determined by χ2 periodogram analysis. Asterisk (*) indicates P < 0.00001, one-way ANOVA with Scheffé post hoc test.
Figure 2
A reduced proportion of _Vip_−/− and _Vipr2_−/− SCN neurons fires rhythmically in vitro. (a) Representative firing rate traces showing examples of circadian and arrhythmic neurons from each genotype. The χ2 periodogram to the left of each plot shows the period estimation of each neuron as in Figure 1. (b) The percentage of SCN neurons with a circadian firing pattern was reduced for both mutants relative to wild-type. (c) The circadian amplitudes (mean ± s.e.m.) of mutant rhythms were lower than in the wild type. Asterisk (*) indicates P < 0.0005, one-way ANOVA with Scheffé post hoc test.
Figure 3
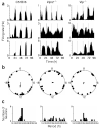
Genetic knockout of Vip or Vipr2 abolishes synchrony among rhythmic SCN neurons in the same culture (a) Representative rhythmic firing rate records from wild-type, _Vipr2_−/− or _Vip_−/− SCN neurons in the same high density culture. Rhythmic neurons in the wild-type culture expressed robust rhythms with similar periods and phases, whereas those in the mutant cultures had variable periods and phases. (b) Relative circadian phases of all rhythmic neurons (n = 24 wild-type, 19 _Vip_−/− and 26 _Vipr2_−/−) from the same three cultures as in a. Data are plotted within circles that represent the fourth day of recording. Arrows indicate the average phase of each distribution. Arrow length reflects the _r_-value of each phase distribution. Wild-type neurons showed statistically significant synchronization (Rayleigh test, P < 0.05, _r_ = 0.36), but _Vipr2_−/− and _Vip_−/− neurons did not (_P_ > 0.7 and P > 0.9, r = 0.09 and 0.05, respectively). (c) Distribution of periods for the same neurons as in b. _Vipr2_−/− and _Vip_−/− SCN neurons showed a significantly broader distribution than did wild-type neurons in the same culture (P < 0.05, Brown-Forsythe's and Levene's tests for equal variance).
Figure 4
The distributions of circadian periods of locomotor activity in mice (left) are similar to those of firing rate rhythms in SCN neurons (right) for the three genotypes. Although the average dominant periods (black bars) of _Vip_−/− and _Vipr2_−/− mutant mice were shorter than those of wild-type mice (P < 0.05, one-way ANOVA with Scheffé _post hoc_ test), the average of all significant circadian periods (gray bars) was similar between genotypes (_P_ > 0.7, Kruskal-Wallis one-way ANOVA with Scheffé post hoc test). Similarly, the average periods of SCN neuronal rhythms did not change with genotype (P > 0.1, Kruskal-Wallis one-way ANOVA). The distributions of all behavioral and firing rate periods were significantly broader in the mutant mice and neurons (P < 0.005 for behavior and P < 0.00005 for neurons, Brown-Forsythe's and Levene's tests for equal variance), indicating a loss of circadian synchrony.
Figure 5
Daily application of VPAC2 agonist Ro 25-1553 restores rhythmicity to _Vip_−/− SCN neurons. (a) Firing rate records of three initially arrhythmic _Vip_−/− neurons and a rhythmic neuron treated with 150 nM Ro 25-1553 every 24 h for 6 d (arrows). The top two traces are from representative neurons in which rhythmicity was restored, and the bottom trace is from a neuron that remained arrhythmic. The third trace is from a representative rhythmic neuron entrained by agonist treatment. Rhythmicity persisted for at least 48 h after the last treatment, indicating that cyclic firing was entrained by drug application. (b) Firing patterns of rhythmic neurons, averaged over the last 4 d of treatment, increased in anticipation of the drug treatment, indicating that the daily change in firing rate was not simply an acute response to the agonist. (c) Relative phases of initially rhythmic (n = 16, left) and arrhythmic neurons with restored rhythms (n = 22, right) from a representative _Vip_−/− culture on the sixth day of Ro 25-1553 treatment. Arrowheads at hour 6 denote the time of agonist application. Both subsets of neurons showed statistically significant synchronization (Rayleigh test, P < 0.05, r = 0.43 and 0.39 for rhythmic and arrhythmic neurons, respectively) with the mean bathyphase of firing (arrows) 10–12 h after agonist application. (d) The broad distribution of periods for rhythmic _Vip_−/− neurons before treatment (left) was significantly narrowed during agonist treatment (right; P < 0.005, Brown-Forsythe's and Levene's tests). (e) The relative proportion of rhythmic (black) to arrhythmic (white) neurons from _Vip_−/− cultures was restored to wild-type levels by daily VPAC2 agonist treatment, but there was little change in the proportion of rhythmic _Vipr2_−/− neurons with the same treatment.
Similar articles
- Disrupted neuronal activity rhythms in the suprachiasmatic nuclei of vasoactive intestinal polypeptide-deficient mice.
Brown TM, Colwell CS, Waschek JA, Piggins HD. Brown TM, et al. J Neurophysiol. 2007 Mar;97(3):2553-8. doi: 10.1152/jn.01206.2006. Epub 2006 Dec 6. J Neurophysiol. 2007. PMID: 17151217 Free PMC article. - The mouse VPAC2 receptor confers suprachiasmatic nuclei cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide in vitro.
Cutler DJ, Haraura M, Reed HE, Shen S, Sheward WJ, Morrison CF, Marston HM, Harmar AJ, Piggins HD. Cutler DJ, et al. Eur J Neurosci. 2003 Jan;17(2):197-204. doi: 10.1046/j.1460-9568.2003.02425.x. Eur J Neurosci. 2003. PMID: 12542655 - Vasoactive intestinal polypeptide (VIP) phase-shifts the rat suprachiasmatic nucleus clock in vitro.
Reed HE, Meyer-Spasche A, Cutler DJ, Coen CW, Piggins HD. Reed HE, et al. Eur J Neurosci. 2001 Feb;13(4):839-43. doi: 10.1046/j.0953-816x.2000.01437.x. Eur J Neurosci. 2001. PMID: 11207820 - The roles of vasoactive intestinal polypeptide in the mammalian circadian clock.
Piggins HD, Cutler DJ. Piggins HD, et al. J Endocrinol. 2003 Apr;177(1):7-15. doi: 10.1677/joe.0.1770007. J Endocrinol. 2003. PMID: 12697032 Review. - An essential role for peptidergic signalling in the control of circadian rhythms in the suprachiasmatic nuclei.
Harmar AJ. Harmar AJ. J Neuroendocrinol. 2003 Apr;15(4):335-8. doi: 10.1046/j.1365-2826.2003.01005.x. J Neuroendocrinol. 2003. PMID: 12622830 Review.
Cited by
- Fibroblast PER2 circadian rhythmicity depends on cell density.
Noguchi T, Wang LL, Welsh DK. Noguchi T, et al. J Biol Rhythms. 2013 Jun;28(3):183-92. doi: 10.1177/0748730413487494. J Biol Rhythms. 2013. PMID: 23735497 Free PMC article. - Synchronization and entrainment of coupled circadian oscillators.
Komin N, Murza AC, Hernández-García E, Toral R. Komin N, et al. Interface Focus. 2011 Feb 6;1(1):167-76. doi: 10.1098/rsfs.2010.0327. Epub 2010 Oct 13. Interface Focus. 2011. PMID: 22419982 Free PMC article. - GRK2: putting the brakes on the circadian clock.
Mendoza-Viveros L, Cheng AH, Cheng HM. Mendoza-Viveros L, et al. Receptors Clin Investig. 2016 Feb 1;3(1):10.14800/rci.1175. doi: 10.14800/rci.1175. Receptors Clin Investig. 2016. PMID: 27088110 Free PMC article. - Molecular components of the Mammalian circadian clock.
Buhr ED, Takahashi JS. Buhr ED, et al. Handb Exp Pharmacol. 2013;(217):3-27. doi: 10.1007/978-3-642-25950-0_1. Handb Exp Pharmacol. 2013. PMID: 23604473 Free PMC article. Review. - Daytime sleep duration during infancy as an indicator for cognitive development at school age: a prospective cohort study.
Lin J, Jiang Y, Xiao X, Zhu Q, Wang G, Lin Q, Jiang F. Lin J, et al. J Clin Sleep Med. 2024 Jul 1;20(7):1069-1077. doi: 10.5664/jcsm.11062. J Clin Sleep Med. 2024. PMID: 38372158
References
- Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 2002;309:109–118. - PubMed
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. - PubMed
- Van Gelder RN, Herzog ED, Schwartz WJ, Taghert PH. Circadian rhythms: in the loop at last. Science. 2003;300:1534–1535. - PubMed
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. - PubMed
- Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat. Neurosci. 1998;1:708–713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH063104/MH/NIMH NIH HHS/United States
- F31 MH073302/MH/NIMH NIH HHS/United States
- MH63104/MH/NIMH NIH HHS/United States
- R01 MH062517/MH/NIMH NIH HHS/United States
- MH073302/MH/NIMH NIH HHS/United States
- MH62517/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases