Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1 - PubMed (original) (raw)
Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1
Makoto Arita et al. J Exp Med. 2005.
Abstract
The essential fatty acid eicosapentaenoic acid (EPA) present in fish oils displays beneficial effects in a range of human disorders associated with inflammation including cardiovascular disease. Resolvin E1 (RvE1), a new bioactive oxygenated product of EPA, was identified in human plasma and prepared by total organic synthesis. Results of bioaction and physical matching studies indicate that the complete structure of RvE1 is 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-EPA. At nanomolar levels, RvE1 dramatically reduced dermal inflammation, peritonitis, dendritic cell (DC) migration, and interleukin (IL) 12 production. We screened receptors and identified one, denoted earlier as ChemR23, that mediates RvE1 signal to attenuate nuclear factor-kappaB. Specific binding of RvE1 to this receptor was confirmed using synthetic [(3)H]-labeled RvE1. Treatment of DCs with small interference RNA specific for ChemR23 sharply reduced RvE1 regulation of IL-12. These results demonstrate novel counterregulatory responses in inflammation initiated via RvE1 receptor activation that provide the first evidence for EPA-derived potent endogenous agonists of antiinflammation.
Figures
Figure 1.
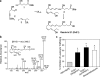
Lipidomic analysis of RvE1 in human plasma. (a) Representative MS/MS selected ion chromatogram at m/z 291 and (b) MS3 selected ion chromatogram at m/z 229. (c) Scheme of RvE1 generation from EPA. Human endothelial cells expressing COX-2 treated with aspirin transform EPA by abstracting hydrogen at C16 to give R insertion of molecular oxygen to yield 18R-H(p)EPE. Alternatively, cytochrome P450 monooxygenase can convert EPA to 18R-HEPE (reference 28). They are further converted via sequential actions of a leukocyte 5-LO–like reaction, which leads to formation of RvE1.
Figure 2.
Properties of RvE1. (a) Total organic synthesis of RvE1. Precursors 1–3 were prepared in isomerically pure form from starting materials with known stereochemistry and coupled sequentially to form acetylenic intermediate compound 4, which was selectively hydrogenated to form isomerically pure RvE1. (b) MS/MS spectrum of synthetic RvE1. (c) Inhibition of leukocyte trafficking in murine dorsal air pouch. 18R-HEPE and RvE1 (biogenic and synthetic) are at 100 ng/mouse, and dexamethasone is at 10 μg/mouse. Values represent mean ± SEM from five different mice. *, P < 0.05 (vs. vehicle control).
Figure 3.
RvE1 receptor. (a) Phylogenetic tree representing amino acid sequence similarities between the human LXA4 receptor (ALX) and related GPCRs. (b) Functional screening for RvE1 receptors. HEK293 cells cotransfected with pNF-κB-luciferase and pcDNA3-GPCRs were exposed to RvE1 (10 nM) and TNF-α. (c) Amino acid sequence alignment of human ChemR23 with ALX. Asterisks indicate conserved amino acids. Putative transmembrane domains are lined and labeled I–VII. (d) RvE1 inhibits luciferase activity in a concentration-dependent manner on cells transfected with pcDNA3-ChemR23 (closed circles) but not pcDNA3 (open circles). (e) Ligand specificity for ChemR23. Cells transfected with pcDNA3-ChemR23 were exposed to 100 nM of each compound. Results are expressed as percent inhibition of luciferase activity and represent the mean ± SEM from n = 3 (b) or n = 4 (d and e); *, P < 0.05. (f) Actions of RvE1 (closed circles), RvE1 derivative (6,14-diacetylenic-RvE1; open rectangle), and 18R-HEPE (closed diamonds) on [35S]-GTP-γS binding to membrane expressing ChemR23. Results are expressed as a percentage of vehicle control with the mean ± SEM (n = 3). *, P < 0.05.
Figure 4.
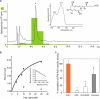
Labeled RvE1 and specific binding. (a) Elution profile of the [3H]-labeled RvE1 on reverse phase HPLC. Tracing was recorded at 270 nm absorbance and chromatographic peak at the same retention time as standard RvE1 is indicated by the asterisk. The inset shows the online UV spectrum of the product. (b) [3H]-RvE1–specific binding to ChemR23. Human ChemR23–transfected (closed circles) or mock-transfected (open inverted triangles) CHO cells (106 cells) were incubated with indicated concentrations of [3H]-RvE1 in the presence or absence of 10 μM of unlabeled RvE1. Saturation curve and Scatchard plot (inset) are representative of n = 3. (c) Binding specificity. Human ChemR23-transfected CHO cells (106 cells) were incubated with 10 nM of [3H]-RvE1 in the presence of 10 μM of RvE1, EPA, 18R-HEPE, or chemerin peptide. Results are expressed as percent competition of [3H]-RvE1–specific binding. Results represent the mean ± SEM from duplicates of n = 3∼5. p-values were obtained by Student's t test comparing heteroligand (i.e., EPA, 18R-HEPE, or chemerin) with homoligand (i.e., RvE1). *, P < 0.01; #, P = 0.07.
Figure 5.
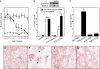
Receptor expression. (a) RT-PCR analysis of human peripheral blood leukocytes and glioma (DBTRG-05MG), monocytic (THP-1), lung epithelial (A549), hepatoma (HepG2), embryonic kidney (HEK293) cell lines, and brain and liver. (b) RT-PCR analysis of human peripheral blood monocytes exposed to either buffer alone, 10 ng/ml TNF-α, or 25 ng/ml IFN-γ for 6 h (gray bars) and 24 h (black bars). Expression levels were quantified by a National Institutes of Health image, normalized by GAPDH levels, and expressed as fold increase over vehicle-treated cells. (c) MAP kinase activation in human peripheral blood monocytic cells were exposed to RvE1 for 5 or 15 min and HEK-ChemR23 cells were treated with 100 nM RvE1 (E) or vehicle (V). (d) Pertussis toxin (PTX) blocks RvE1-induced ERK activation and NF-κB inhibition in HEK-ChemR23 cells. (e) EAR with HEK-ChemR23 cells exposed to vehicle (red line), 100 nM RvE1 (blue line), 10 μM chemerin peptide, or 10 μM ATP using Cytosensor microphysiometer. These responses were observed from three separate experiments. (f) Actions of RvE1 (red) and chemerin peptide (black) on [35S]-GTP-γS binding to HEK293 cell membranes expressing human ChemR23. (g) Inhibition of TNF-α induced NF-κB luciferase activities by RvE1 (red) and chemerin peptide (black) on HEK293 cells expressing human ChemR23.
Figure 6.
RvE1 regulates DCs. (a) RvE1 inhibits DC IL-12 production in vitro stimulated by pathogen extract (STAg). CD11c+ DCs incubated with vehicle (open circles) or RvE1 (closed circles) before STAg or no STAg (open squares). (b) Reduction of ChemR23 expression by siRNA eliminates RvE1 signaling. Expression of ChemR23 and GAPDH mRNA from DCs treated with either scramble or ChemR23-specific siRNAs (inset). Spleen cells transfected with siRNAs were treated with vehicle (ethanol, 0.1% vol/vol) or RvE1 (1.0 μg/ml). 8 h later, cells were stimulated with 10 μg/ml STAg and IL-12p40 was measured. Bars represent mean ± SD (n = 3), *, P < 0.05 (control vs. specific siRNA). (c) RvE1 blocks IL-12 production in vivo. Mice administered with either 100 ng RvE1 or vehicle were challenged intraperitoneally with PBS or STAg, and IL-12p40 secretion from splenic CD11c+ DCs was measured. (d–g) RvE1 blocks trafficking of CD11c+ DCs in spleen with pathogen extract challenge. Spleens from mice given 10 μg RvE1 or vehicle were stained for CD11c. (d) PBS plus vehicle (e) STAg plus vehicle (f) PBS plus RvE1 (g) STAg plus RvE1. Arrows indicate CD11c positive DCs accumulated in T cell–enriched area.
Similar articles
- Resolvin E1 receptor activation signals phosphorylation and phagocytosis.
Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Ohira T, et al. J Biol Chem. 2010 Jan 29;285(5):3451-61. doi: 10.1074/jbc.M109.044131. Epub 2009 Nov 11. J Biol Chem. 2010. PMID: 19906641 Free PMC article. - Omega-3 fatty acid-derived mediator, Resolvin E1, ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice.
Kim TH, Kim GD, Jin YH, Park YS, Park CS. Kim TH, et al. Int Immunopharmacol. 2012 Dec;14(4):384-91. doi: 10.1016/j.intimp.2012.08.005. Epub 2012 Aug 27. Int Immunopharmacol. 2012. PMID: 22951188 - Identification of endogenous resolvin E1 and other lipid mediators derived from eicosapentaenoic acid via electrospray low-energy tandem mass spectrometry: spectra and fragmentation mechanisms.
Lu Y, Hong S, Yang R, Uddin J, Gotlinger KH, Petasis NA, Serhan CN. Lu Y, et al. Rapid Commun Mass Spectrom. 2007;21(1):7-22. doi: 10.1002/rcm.2798. Rapid Commun Mass Spectrom. 2007. PMID: 17131464 - The contributions of aspirin and microbial oxygenase to the biosynthesis of anti-inflammatory resolvins: novel oxygenase products from omega-3 polyunsaturated fatty acids.
Arita M, Clish CB, Serhan CN. Arita M, et al. Biochem Biophys Res Commun. 2005 Dec 9;338(1):149-57. doi: 10.1016/j.bbrc.2005.07.181. Epub 2005 Aug 10. Biochem Biophys Res Commun. 2005. PMID: 16112645 Review. - Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis.
Serhan CN, Gotlinger K, Hong S, Arita M. Serhan CN, et al. Prostaglandins Other Lipid Mediat. 2004 Apr;73(3-4):155-72. doi: 10.1016/j.prostaglandins.2004.03.005. Prostaglandins Other Lipid Mediat. 2004. PMID: 15290791 Review.
Cited by
- Serum resolvin E1 levels and its relationship with thyroid autoimmunity in Hashimoto's thyroiditis: a preliminary study.
Song J, Sun R, Zhang Y, Ke J, Zhao D. Song J, et al. BMC Endocr Disord. 2021 Apr 13;21(1):66. doi: 10.1186/s12902-021-00730-9. BMC Endocr Disord. 2021. PMID: 33849519 Free PMC article. - Chemerin: a potential endocrine link between obesity and type 2 diabetes.
Roman AA, Parlee SD, Sinal CJ. Roman AA, et al. Endocrine. 2012 Oct;42(2):243-51. doi: 10.1007/s12020-012-9698-8. Epub 2012 May 19. Endocrine. 2012. PMID: 22610747 Review. - Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid.
Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, Inoue M, Arai H. Isobe Y, et al. J Biol Chem. 2012 Mar 23;287(13):10525-10534. doi: 10.1074/jbc.M112.340612. Epub 2012 Jan 24. J Biol Chem. 2012. PMID: 22275352 Free PMC article. - Resolvin E1 maintains macrophage function under cigarette smoke-induced oxidative stress.
Takamiya R, Fukunaga K, Arita M, Miyata J, Seki H, Minematsu N, Suematsu M, Asano K. Takamiya R, et al. FEBS Open Bio. 2012 Oct 17;2:328-33. doi: 10.1016/j.fob.2012.10.001. Print 2012. FEBS Open Bio. 2012. PMID: 23772366 Free PMC article. - Intravenous fish oil lipid emulsions in critically ill patients: an updated systematic review and meta-analysis.
Manzanares W, Langlois PL, Dhaliwal R, Lemieux M, Heyland DK. Manzanares W, et al. Crit Care. 2015 Apr 16;19(1):167. doi: 10.1186/s13054-015-0888-7. Crit Care. 2015. PMID: 25879776 Free PMC article. Review.
References
- De Caterina, R., S. Endres, S.D. Kristensen, and E.B. Schmidt. 1993. N-3 Fatty Acids and Vascular Disease. Bi & Gi Publishers, Verona. 166 pp.
- GISSI-Prevenzione Investigators. 1999. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 354:447–455. - PubMed
- Albert, C.M., H. Campos, M.J. Stampfer, P.M. Ridker, J. Manson, W.C. Willett, and J. Ma. 2002. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N. Engl. J. Med. 346:1113–1118. - PubMed
- Weiss, U. 2002. Insight: inflammation. Nature. 420:845–891.
- Funk, C.D. 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 294:1871–1875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM38765/GM/NIGMS NIH HHS/United States
- R01 GM038765/GM/NIGMS NIH HHS/United States
- P50- DE-016191/DE/NIDCR NIH HHS/United States
- P50 DE016191/DE/NIDCR NIH HHS/United States
- R37 GM038765/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials