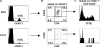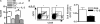Essential role for interleukin-2 for CD4(+)CD25(+) T regulatory cell development during the neonatal period - PubMed (original) (raw)
Essential role for interleukin-2 for CD4(+)CD25(+) T regulatory cell development during the neonatal period
Allison L Bayer et al. J Exp Med. 2005.
Abstract
Although many aspects of CD4(+)CD25(+) T regulatory (T(reg)) cell development remain largely unknown, signaling through the IL-2R represents one feature for the production of T(reg) cells. Therefore, the present study was undertaken to further define early developmental steps in the production of T(reg) cells, including a more precise view on the role of interleukin (IL)-2 in this process. After adoptive transfer of wild-type T(reg) cells into neonatal IL-2Rbeta(-/-) mice, only a small fraction of donor T(reg) cells selectively seeded the lymph node (LN). These donor T(reg) cells underwent rapid and extensive IL-2-dependent proliferation, followed by subsequent trafficking to the spleen. Thus, IL-2 is essential for T(reg) cell proliferation in neonatal LN. The number and distribution of T(reg) cells in the periphery of normal neonatal mice closely paralleled that seen for IL-2Rbeta(-/-) mice that received T(reg) cells. However, for normal neonates, blockade of IL-2 decreased T(reg) cells in both the thymus and LN. Therefore, two steps of T(reg) cell development depend upon IL-2 in neonatal mice, thymus production, and subsequent expansion in the LN.
Figures
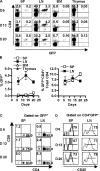
Figure 1.
Initial appearance of CD4+CD25+ Treg cells in the lymph nodes of neonatal mice. Newborn C57BL/6 IL-2Rβ−/− mice received purified GFP-CD4+CD25+ T cells (4–5 × 105) and 6–20 d after transfer, spleen (SP), lymph node (LN), bone marrow (BM), and thymus cells from individual recipient mice were subjected to FACS analysis. (A) Dot plots for donor cells based on CD4 and GFP expression. The percent of cells expressing CD4 and/or GFP is indicated in appropriate quadrant. (B) The percent of donor GFP+ cells (left) or donor GFP+CD4+ of the total CD4+ cells (right) in the indicated organ. Data are mean ± SEM for three to five animals per day. (C) Dot plots for CD4 and CD8 expression for GFP+ donor cells. The percent of cells expressing CD4 or CD8 is indicated in the appropriate quadrant. (D) CD25 expression for CD4+ GFP+ donor cells. The percent of cells expressing CD25 is indicated in the upper right corner of each histogram. Data in A, C, and D are representative of three to five animals per day.
Figure 2.
CD4+CD25+ Treg cells extensively proliferate in the lymph nodes of neonatal mice. Newborn C57BL/6 IL-2Rβ−/− mice received purified CD45.1+ CFSE-labeled CD4+CD25+ T cells (4–5 × 105) and 2–20 d after transfer, spleen (SP), and lymph node (LN) cells from individual recipient mice were subjected to FACS analysis. (A) Dot plots are shown for CD45.1 and CD4 expression (top) or CD25 and CFSE expression for CD45.1+CD4+ donor cells (bottom). The percent of donor CD4+ cells is indicated in the upper right corner of top dot plots. A total of 105 or 106 splenic cells were collected from 2- or 4-d-old mice, respectively. (B) Dot plots are shown for CD45.1 and CFSE expression. The percent of donor cells is indicated in the upper right corner of each dot plot. (C) Dot plots depicting CD4 and CD25 expression for CD45.1+ donor cells. The percent of cells expressing CD4 and CD25 of the gated populations is indicated in the upper right corner of the dot plots. Data in A and B are representative of two to five animals per day.
Figure 3.
Adoptive transfer of Treg cells into IL-2Rβ2/− mice largely follows the developmental scheme operative in normal mice. FACS analysis of lymph node and spleen cells was performed for 6–20-d-old mice, as indicated. The percent CD4- and CD25-positive cells (left) or the absolute number of CD4+CD25+ (right) in the (A) lymph node and (B) spleen. The absolute number of CD4+CD25+ T cells was calculated from the percent CD4+CD25+ cells in the lymph node and spleen times the number of cells in these organs. Data are mean ± SEM for three to seven mice per day per group. (C) CD69, CD62L, or CD103 expression for lymph node CD4+CD25+ T cells (n = 3–5 animals per group per day).
Figure 4.
The initial engraftment and proliferation of Treg cells in neonatal lymph nodes requires IL-2. Neonatal IL-2Rβ−/− mice were adoptively transferred with CD45.1+ CFSE-labeled CD4+CD25+ T cells (2 × 105) and received either PBS or α-IL-2 mAb (100 μg) on the day of transfer and 3 d later. 6 d after transfer, lymph node cells from individual recipient mice were subjected to FACS analysis. (A) Histograms of donor (CD45.1) engraftment. (B) Dot plots for CD4 and CD25 expression after gating on CD45.1+ donor cells. (C) Histograms of proliferating donor CD4+CD25+ T cells based on CFSE staining. n = 3 per group.
Figure 5.
A Role for IL-2 in early development of CD4+CD25+ Treg cells. B6 mice received either PBS (−) or α-IL2 mAb (+; 100 μg) on the day and 3 d after birth. 7 d after birth, lymph node cells from individual mice were subjected to FACS analysis. (A) The percent of CD4+CD25+ T cells. Data are mean ± SEM for 8–10 animals. (B) Dot plots of CD25 and GITR expression (left) and the absolute number of GITR+ cells for CD4+CD25+ cells (right). Data were mean ± SEM for three to four animals.
Figure 6.
IL-2 is required for thymic production of CD4+CD25+ Treg cells. All mice received either PBS (−) or α-IL2 mAb (+; 100 μg) on the day and 3 d after birth. 7 d after birth, thymocytes from individual mice were subjected to FACS analysis with 250,000 events collected. In the data shown, the analysis of the indicated markers was performed after gating on CD4+CD8− “single positive” thymocytes. (A) The percent of thymic CD4+CD25+ cells from B6 and Tg−/− treated mice. Data are mean ± SEM for six to eight animals per group. *P < 0.05 compared with B6, and #P < 0.001 compared with Tg−/− (one-way analysis of variance followed by Newman-Keuls Multiple Comparison). (B) Dot plots for CD25 and GITR expression for thymic CD4 single positive thymocytes from B6 mice (left) and the absolute number of GITR+ CD4 single positive thymocytes (right). Data are mean ± SEM for three to four animals per group.
Similar articles
- CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2.
Malek TR, Yu A, Vincek V, Scibelli P, Kong L. Malek TR, et al. Immunity. 2002 Aug;17(2):167-78. doi: 10.1016/s1074-7613(02)00367-9. Immunity. 2002. PMID: 12196288 - Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells.
Bayer AL, Yu A, Malek TR. Bayer AL, et al. J Immunol. 2007 Apr 1;178(7):4062-71. doi: 10.4049/jimmunol.178.7.4062. J Immunol. 2007. PMID: 17371960 - CD40/CD40L interaction regulates CD4+CD25+ T reg homeostasis through dendritic cell-produced IL-2.
Guiducci C, Valzasina B, Dislich H, Colombo MP. Guiducci C, et al. Eur J Immunol. 2005 Feb;35(2):557-67. doi: 10.1002/eji.200425810. Eur J Immunol. 2005. PMID: 15682445 - T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells.
Fahlén L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. Fahlén L, et al. J Exp Med. 2005 Mar 7;201(5):737-46. doi: 10.1084/jem.20040685. J Exp Med. 2005. PMID: 15753207 Free PMC article. - Human CD4+CD25+ regulatory T cells.
Baecher-Allan C, Viglietta V, Hafler DA. Baecher-Allan C, et al. Semin Immunol. 2004 Apr;16(2):89-98. doi: 10.1016/j.smim.2003.12.005. Semin Immunol. 2004. PMID: 15036232 Review.
Cited by
- Immunogenetics of autoimmune thyroid diseases: A comprehensive review.
Lee HJ, Li CW, Hammerstad SS, Stefan M, Tomer Y. Lee HJ, et al. J Autoimmun. 2015 Nov;64:82-90. doi: 10.1016/j.jaut.2015.07.009. Epub 2015 Jul 30. J Autoimmun. 2015. PMID: 26235382 Free PMC article. Review. - Dendritic cells in the periphery control antigen-specific natural and induced regulatory T cells.
Yamazaki S, Morita A. Yamazaki S, et al. Front Immunol. 2013 Jun 21;4:151. doi: 10.3389/fimmu.2013.00151. eCollection 2013. Front Immunol. 2013. PMID: 23801989 Free PMC article. - Development of regulatory T cells requires IL-7Ralpha stimulation by IL-7 or TSLP.
Mazzucchelli R, Hixon JA, Spolski R, Chen X, Li WQ, Hall VL, Willette-Brown J, Hurwitz AA, Leonard WJ, Durum SK. Mazzucchelli R, et al. Blood. 2008 Oct 15;112(8):3283-92. doi: 10.1182/blood-2008-02-137414. Epub 2008 Jul 29. Blood. 2008. PMID: 18664628 Free PMC article. - CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells.
Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. Yamazaki S, et al. J Immunol. 2008 Nov 15;181(10):6923-33. doi: 10.4049/jimmunol.181.10.6923. J Immunol. 2008. PMID: 18981112 Free PMC article. - Apoptosis of CD4+ CD25(high) T cells in type 1 diabetes may be partially mediated by IL-2 deprivation.
Jailwala P, Waukau J, Glisic S, Jana S, Ehlenbach S, Hessner M, Alemzadeh R, Matsuyama S, Laud P, Wang X, Ghosh S. Jailwala P, et al. PLoS One. 2009 Aug 5;4(8):e6527. doi: 10.1371/journal.pone.0006527. PLoS One. 2009. PMID: 19654878 Free PMC article.
References
- Shevach, E.M. 2000. Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 18:423–449. - PubMed
- Sakaguchi, S. 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 101:455–458. - PubMed
- Jordan, M.S., A. Boesteanu, A.J. Reed, A.L. Petrone, A.E. Holenbeck, M.A. Lerman, A. Naji, and A.J. Caton. 2001. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2:301–306. - PubMed
- Apostolou, I., A. Sarukhan, L. Klein, and H. von Boehmer. 2002. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 3:756–763. - PubMed
- Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI055815/AI/NIAID NIH HHS/United States
- R01 CA045957/CA/NCI NIH HHS/United States
- AI055815/AI/NIAID NIH HHS/United States
- AI 40114/AI/NIAID NIH HHS/United States
- R01 AI040114/AI/NIAID NIH HHS/United States
- R56 AI040114/AI/NIAID NIH HHS/United States
- CA45957/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials