Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice - PubMed (original) (raw)
Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice
Alexander Swidsinski et al. World J Gastroenterol. 2005.
Abstract
Aim: To study the role of intestinal flora in inflammatory bowel disease (IBD).
Methods: The spatial organization of intestinal flora was investigated in normal mice and in two models of murine colitis using fluorescence in situ hybridization.
Results: The murine small intestine was nearly bacteria-free. The normal colonic flora was organized in three distinct compartments (crypt, interlaced, and fecal), each with different bacterial compositions. Crypt bacteria were present in the cecum and proximal colon. The fecal compartment was composed of homogeneously mixed bacterial groups that directly contacted the colonic wall in the cecum but were separated from the proximal colonic wall by a dense interlaced layer. Beginning in the middle colon, a mucus gap of growing thickness physically separated all intestinal bacteria from contact with the epithelium. Colonic inflammation was accompanied with a depletion of bacteria within the fecal compartment, a reduced surface area in which feces had direct contact with the colonic wall, increased thickness and spread of the mucus gap, and massive increases of bacterial concentrations in the crypt and interlaced compartments. Adhesive and infiltrative bacteria were observed in inflamed colon only, with dominant Bacteroides species.
Conclusion: The proximal and distal colons are functionally different organs with respect to the intestinal flora, representing a bioreactor and a segregation device. The highly organized structure of the colonic flora, its specific arrangement in different colonic segments, and its specialized response to inflammatory stimuli indicate that the intestinal flora is an innate part of host immunity that is under complex control.
Figures
Figure 1
I = ileum of a wild type normal mouse narrow and free of bacteria in most parts. E = epithelium; L = lumen.
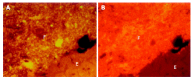
Figure 2
Concentrated bacterial mass in direct contact with the mucosal surface (A) and non-adherent bacteria (B) in cecum of a healthy wild type mouse. E = epithelium; F = feces. Arrow indicates the shrinkage of feces, arrowheads indicate bacteria in crypts.
Figure 3
Interlaced layer in the distal portion of the proximal colon.
Figure 4
Middle colon in a normal mouse.
Figure 5
Rectal mucosa covered with thick mucus (M). E = epithelium, F = feces.
Figure 6
Fecal bacteria hybridized with Bacteroides (Cy3 green-orange, 6A) and Erec (Cy5 red, 6B) probes, cecum of healthy WT mice.
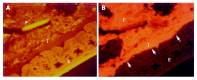
Figure 7
Interlaced layer in the proximal colon of IL-10 mice with colitis visualized simultaneously by hybridization with Bac303 (Cy3 green-orange, 7A) and Eub338 probes (Cy5 red, 7B).
Figure 8
Interlaced layer in proximal colon of DSS mice visualized simultaneously by hybridization with Clit135 (Cy5 red, 8A) and Lab158 probes (Cy3 green-orange, 8B). I = interlaced layer, E = epithelium, F = feces.
Figure 9
Bacteria location below the intact mucus layer (arrowheads) and adherence to the colonic mucosa in DSS-exposed mice.
Figure 10
Bacteria infiltrating the mucosa in distal colon of IL-10 mice with colitis (arrowheads).
Similar articles
- Azathioprine and mesalazine-induced effects on the mucosal flora in patients with IBD colitis.
Swidsinski A, Loening-Baucke V, Bengmark S, Lochs H, Dörffel Y. Swidsinski A, et al. Inflamm Bowel Dis. 2007 Jan;13(1):51-6. doi: 10.1002/ibd.20003. Inflamm Bowel Dis. 2007. PMID: 17206639 - Intestinal mucus barrier in normal and inflamed colon.
Corazziari ES. Corazziari ES. J Pediatr Gastroenterol Nutr. 2009 Apr;48 Suppl 2:S54-5. doi: 10.1097/MPG.0b013e3181a117ea. J Pediatr Gastroenterol Nutr. 2009. PMID: 19300126 - Comparative study of the intestinal mucus barrier in normal and inflamed colon.
Swidsinski A, Loening-Baucke V, Theissig F, Engelhardt H, Bengmark S, Koch S, Lochs H, Dörffel Y. Swidsinski A, et al. Gut. 2007 Mar;56(3):343-50. doi: 10.1136/gut.2006.098160. Epub 2006 Aug 14. Gut. 2007. PMID: 16908512 Free PMC article. - The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics.
Linskens RK, Huijsdens XW, Savelkoul PH, Vandenbroucke-Grauls CM, Meuwissen SG. Linskens RK, et al. Scand J Gastroenterol Suppl. 2001;(234):29-40. doi: 10.1080/003655201753265082. Scand J Gastroenterol Suppl. 2001. PMID: 11768558 Review. - Role of bacteria in experimental colitis.
Guarner F, Malagelada JR. Guarner F, et al. Best Pract Res Clin Gastroenterol. 2003 Oct;17(5):793-804. doi: 10.1016/s1521-6918(03)00068-4. Best Pract Res Clin Gastroenterol. 2003. PMID: 14507589 Review.
Cited by
- Association Between Sex Hormone Levels and Gut Microbiota Composition and Diversity-A Systematic Review.
d'Afflitto M, Upadhyaya A, Green A, Peiris M. d'Afflitto M, et al. J Clin Gastroenterol. 2022 May-Jun 01;56(5):384-392. doi: 10.1097/MCG.0000000000001676. J Clin Gastroenterol. 2022. PMID: 35283442 Free PMC article. - Signal transducer and activator of transcription 5b promotes mucosal tolerance in pediatric Crohn's disease and murine colitis.
Han X, Osuntokun B, Benight N, Loesch K, Frank SJ, Denson LA. Han X, et al. Am J Pathol. 2006 Dec;169(6):1999-2013. doi: 10.2353/ajpath.2006.060186. Am J Pathol. 2006. PMID: 17148664 Free PMC article. - Altered Profiles of Gut Microbiota in Klebsiella pneumoniae-Induced Pyogenic Liver Abscess.
Chen N, Ling ZX, Jin TT, Li M, Zhao S, Zheng LS, Xi X, Wang LL, Chen YY, Shen YL, Zhang LP, Sun SC. Chen N, et al. Curr Microbiol. 2018 Jul;75(7):952-959. doi: 10.1007/s00284-018-1471-7. Epub 2018 Apr 10. Curr Microbiol. 2018. PMID: 29637226 - Intestinal microbiota in pathophysiology and management of irritable bowel syndrome.
Lee KN, Lee OY. Lee KN, et al. World J Gastroenterol. 2014 Jul 21;20(27):8886-97. doi: 10.3748/wjg.v20.i27.8886. World J Gastroenterol. 2014. PMID: 25083061 Free PMC article. Review. - Heterogeneity across the murine small and large intestine.
Bowcutt R, Forman R, Glymenaki M, Carding SR, Else KJ, Cruickshank SM. Bowcutt R, et al. World J Gastroenterol. 2014 Nov 7;20(41):15216-32. doi: 10.3748/wjg.v20.i41.15216. World J Gastroenterol. 2014. PMID: 25386070 Free PMC article. Review.
References
- Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5S–11S. - PubMed
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous