Diversifying carotenoid biosynthetic pathways by directed evolution - PubMed (original) (raw)
Review
Diversifying carotenoid biosynthetic pathways by directed evolution
Daisuke Umeno et al. Microbiol Mol Biol Rev. 2005 Mar.
Abstract
Microorganisms and plants synthesize a diverse array of natural products, many of which have proven indispensable to human health and well-being. Although many thousands of these have been characterized, the space of possible natural products--those that could be made biosynthetically--remains largely unexplored. For decades, this space has largely been the domain of chemists, who have synthesized scores of natural product analogs and have found many with improved or novel functions. New natural products have also been made in recombinant organisms, via engineered biosynthetic pathways. Recently, methods inspired by natural evolution have begun to be applied to the search for new natural products. These methods force pathways to evolve in convenient laboratory organisms, where the products of new pathways can be identified and characterized in high-throughput screening programs. Carotenoid biosynthetic pathways have served as a convenient experimental system with which to demonstrate these ideas. Researchers have mixed, matched, and mutated carotenoid biosynthetic enzymes and screened libraries of these "evolved" pathways for the emergence of new carotenoid products. This has led to dozens of new pathway products not previously known to be made by the assembled enzymes. These new products include whole families of carotenoids built from backbones not found in nature. This review details the strategies and specific methods that have been employed to generate new carotenoid biosynthetic pathways in the laboratory. The potential application of laboratory evolution to other biosynthetic pathways is also discussed.
Figures
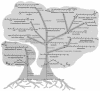
FIG. 1.
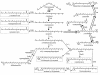
Natural carotenoid biosynthetic pathways can be organized in a tree-like hierarchy. The biosynthesis of all natural carotenoids begins with the enzymatic assembly of a C30 or C40 backbone. These backbones are desaturated, cyclized, oxidized, and otherwise modified by downstream enzymes in various species-specific combinations. Shown are several common types of enzymatic transformations that occur in natural carotenoid pathways. Common carotenoids formed early in a pathway, such as lycopene, are modified in different organisms depending on the enzymes present. The multiple enzymatic routes originating from intermediates common to many end products result in extensive pathway branching (and subbranching).
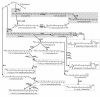
FIG. 2.
Selected examples of gene assembly leading to novel carotenoids in E. coli. Combining enzymes from different source organisms can lead to rare or novel carotenoids. In some cases, such as with CrtC and CrtD (4, 5), new pathway branches can be uncovered by replacing an enzyme with its counterpart from a different organism (192). Carotenoids in boxes had not been previously identified in biological material or chemically synthesized. Enzyme abbreviations: Al-1, phytoene desaturase from Neurospora crassa (five-step); CrtC, neurosporene 1,2-hydratase; CrtD, 1-OH-neurosporene 3,4-desaturase; CrtI_Eu_, phytoene desaturase from Erwinia uredovora (four-step); CrtI_Rc_, phytoene desaturase from Rhodobacter capsulatus (three-step); CrtW, β,β-carotene 4,(4′)-ketolase; CrtX, zeaxanthin glycosylase; CrtY, lycopene β-cyclase; CrtZ, β,β-carotene 3,(3′)-hydroxylase. Other enzyme subscripts: Aa, “Agrobacterium aurantiacum” (current designation, Paracoccus sp. strain MBIC1143); Eu, Erwinia uredovora (current approved name, Pantoea ananatis); Rg, Rubrivivax gelatinosus; Rs, Rhodobacter sphaeroides. Natural pathways leading to the synthesis of demethylspheroidene and β,β-carotene are shaded. The dotted arrow represents trace enzymatic conversion, and the crossed-out arrow denotes undetectable enzymatic conversion. References to original publications describing the biosynthesis of various compounds are given in brackets beside compound names.
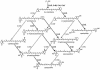
FIG. 3.
“Matrix” pathway resulting from the coexpression of carotenoid biosynthetic enzymes with broad specificity. Coexpression in E. coli of CrtW [β,β-carotene 4,(4′)-ketolase] and CrtZ [β,β-carotene 3,(3′)-hydroxylase] from “_Agrobacterium aurantiacum_” (current designation, Paracoccus sp. strain MBIC1143) along with the genes for β,β-carotene synthesis from E. uredovora (current approved name, Pantoea ananatis) resulted in the biosynthesis of at least nine different β,β-carotene derivatives (135). This figure was made using data from reference .
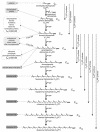
FIG. 4.
Chain elongation reactions catalyzed by natural all-E IDSs. Each IDS adds a defined number of IPP molecules to an allylic diphosphate substrate before the final product is released. Wild-type IDSs have quite stringent product specificity; catalytic ranges of known all-E IDSs are shown with arrows on the right. Natural products derived from the various isoprenyl diphosphates are shown on the left. This figure was made using data from reference .
FIG. 5.
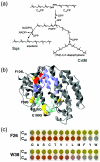
Product length determination by CrtM and mutants thereof. (a) Reaction mechanism of squalene synthase (left pathway) and CrtM (right pathway). Both enzymes catalyze the head-to-head condensation of two molecules of C15PP to yield linear C30 hydrocarbons. The enzymes employ similar mechanisms involving a common stable intermediate, presqualene diphosphate (PSPP). The mechanisms differ only in the final rearrangement step, which is followed in the case of squalene synthase by reduction of the central double bond. (b) Crystal structure of human squalene synthase (PDB ID 1EZF). Green residues are involved in the first half-reaction (formation of PSPP), while blue residues form the pocket of the second half-reaction in which PSPP is rearranged. Red residues correspond by sequence alignment to F26 and W38 of CrtM, which were found to control the product size of that enzyme (213, 214). Shown in yellow are the residues that align with mutations in CrtM that improve both its C30 and C40 carotenoid synthase activities. (c) Mutation analysis of F26 and W38 of CrtM. Various site-directed variants of CrtM (indicated by their one-letter amino acid code) were constructed and tested for their synthase activity in both C30 and C40 carotenoid pathways. Variants are arranged from left to right in increasing order with respect to the van der Waals' volume of the substituted amino acid. The C30 carotenoid synthase activity of each variant was estimated from the level of yellow pigmentation when coexpressed with CrtN. Similarly, the C40 carotenoid synthase function of each variant was evaluated in terms of the level of red pigmentation when coexpressed with CrtE and CrtI. Panels a and b were made using data from reference .
FIG. 6.
Extension of laboratory-evolved pathways to 3,4,3′,4′-tetradehydrolycopene and torulene by coexpression of downstream carotenoid modifying enzymes. Boxed structures had not been previously identified in biological material. Native enzyme reactions are shown with black arrows. Gray arrows depict reactions not seen in natural organisms, and laboratory-evolved enzymes are written in gray lettering. Double arrows indicate enzymatic modification of both ends of a carotenoid substrate. Enzyme abbreviations: CrtA, spheroidene monooxygenase; CrtI, phytoene desaturase from E. uredovora (current approved name, Pantoea ananatis) (four-step), CrtI14, laboratory-evolved mutant of CrtI (four- to six-step) (180); CrtO, β,β-carotene 4,(4′)-ketolase; CrtU, β,β-carotene desaturase; CrtX, zeaxanthin glycosylase; CrtY, lycopene β-cyclase; CrtY2, laboratory-evolved mutant of CrtY (180); CrtZ, β,β-carotene 3,(3′)-hydroxylase. Biosynthesis of oxygenated derivatives of ζ-carotene and neurosporene was attributed to early termination by CrtA of the desaturation sequence of CrtI, which is normally a four-step desaturase (118). Although CrtA is primarily a ketolase, it is thought to catalyze the introduction of both hydroxy groups of phillipsiaxanthin (C. Schmidt-Dannert, personal communication). This figure was made using data from reference .
FIG. 7.
The step number of the C30 desaturase CrtN is readily modified by mutation. A library of mutant crtN alleles was coexpressed with the C30 carotenoid synthase crtM in E. coli. Wild-type CrtN is a three- to four-step desaturase when expressed in E. coli, causing colonies to appear orange (inset). Colonies harboring CrtN mutants with increased step number were deep orange and red (highlighted by arrows), reflecting the longer carotenoid chromophores formed by these mutant desaturases. Colonies harboring mutants with decreased step number appear yellow/lemon, while colonies with nonfunctional or single-step desaturases are pale since these products are colorless. Shown below are the possible C30 desaturation products and the desaturases that synthesize them. CrtN1-3 are discovered mutants of CrtN with altered desaturation step number. Each colored box depicts the approximate color of the carotenoid in white light. Molecules without colored boxes are colorless. Abbreviations: lacP, lac promoter; wt, wild type.
FIG. 8.
Cyclization of carotenoids. (a) Mechanism of carotenoid cyclization. Lycopene with its two ψ-end groups is the preferred substrate for all known carotenoid cyclases. All cyclases produce the same carbocationic intermediate on protonation of C-2 of the substrate. Product ring type (β, ɛ, or γ) is determined by which proton (a, b, or c in the figure) is eliminated in the subsequent rearrangement step. This panel was made using data from reference . (b) Substrates accepted by lycopene cyclases. The shaded portion(s) of each structure is cyclized. The first three compounds, with their ψ-end groups, have long been known as natural substrates for lycopene cyclases. The remaining seven structures, including neurosporene with its 7,8-dihydro-ψ-end (left side of molecule) and ζ-carotene with two 7,8-dihydro-ψ-ends, have more recently been shown to be cyclized.
FIG. 9.
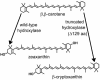
Alteration of product specificity of the β,β-carotene hydroxylase from Arabidopsis thaliana. The wild-type enzyme catalyzes two hydroxylation steps, converting β,β-carotene to zeaxanthin when expressed in either A. thaliana or E. coli. When the N-terminal 129 amino acids (aa) were removed, the truncated enzyme expressed in E. coli catalyzed only one hydroxylation step, leading primarily to synthesis of β-cryptoxanthin (197).
FIG. 10.
Several carotenoid cleavage enzymes display localized specificity. Gray arrowheads show bonds cleaved. (a) β,β-Carotene-15,15′-monooxygenase (βCM) can cleave substrates with an unsubstituted β-end (123, 228). (b) Murine β,β-carotene-9′,10′-oxygenase (βCO9-10) can also cleave lycopene, leading to uncharacterized apolycopenals (98). (c) One carotenoid cleavage dioxygenase from A. thaliana (_At_CCD1) cleaves at least six different carotenoid substrates, leading to a variety of products (185). The downward arrow from C14 dialdehyde indicates that this product was detected from all six substrates shown.
FIG. 11.
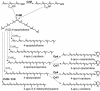
Generation of a C35 carotenoid pathway by gene assembly and directed enzyme evolution (211). When supplied with C15PP and C20PP, the C30 carotenoid synthase CrtM produces the C35 carotenoid backbone, 4-apophytoene. By coexpressing C30 or C40 desaturases and mutants thereof (gray), different clones could be found that produce each possible acyclic C35 carotenoid as the main product. E. uredovora (P. ananatis) β-cyclase (CrtY) and L. sativa ɛ-cyclase (Dy4) were shown to cyclize two of the C35 substrates, leading to four different cyclic C35 carotenoids. All 10 C35 carotenoids in this figure had never been previously reported.
FIG. 12.
Biosynthesis of carotenoids with C45 and C50 backbones (212). (a) Expression of the Y81A variant of the C15PP synthase from B. stearothermophilus (BstFPSY81A) led to the production of C20PP and C25PP in E. coli. Additional coexpression of variants of the C30 carotenoid synthase CrtM mutated at F26 and/or W38 (CrtMmut) resulted in biosynthesis of the C45 carotenoid backbone 16-isopentenylphytoene (C20 + C25) and the C50 backbone 16,16′-diisopentenylphytoene (C25 + C25). Both of these larger carotenoids had never been previously reported. (b) E. coli cells expressing BstFPSY81A, CrtMF26A,W38A, and the C40 desaturase CrtI accumulated as yet uncharacterized desaturated carotenoids with a deep red color (third row). Shown for color comparison is the acetone extract of an E. coli culture expressing CrtM and CrtN and synthesizing the C30 carotenoids 4,4′-diaponeurosporene and 4,4′-diapolycopene (first row) and the acetone extract of an E. coli culture expressing CrtE, CrtB, and CrtI and synthesizing the C40 carotenoids lycopene and 3,4,3′,4′-tetradehydrolycopene (second row). Abbreviations: lacP, lac promoter; Eu, Erwinia uredovora (current approved name, Pantoea ananatis).
Similar articles
- Molecular breeding of carotenoid biosynthetic pathways.
Schmidt-Dannert C, Umeno D, Arnold FH. Schmidt-Dannert C, et al. Nat Biotechnol. 2000 Jul;18(7):750-3. doi: 10.1038/77319. Nat Biotechnol. 2000. PMID: 10888843 - Use of directed enzyme evolution to create novel biosynthetic pathways for production of rare or non-natural carotenoids.
Furubayashi M, Umeno D. Furubayashi M, et al. Methods Enzymol. 2022;671:351-382. doi: 10.1016/bs.mie.2022.03.008. Epub 2022 Apr 18. Methods Enzymol. 2022. PMID: 35878986 - Engineering novel carotenoids in microorganisms.
Schmidt-Dannert C. Schmidt-Dannert C. Curr Opin Biotechnol. 2000 Jun;11(3):255-61. doi: 10.1016/s0958-1669(00)00093-8. Curr Opin Biotechnol. 2000. PMID: 10851142 Review. - Identification of a carotenoid oxygenase synthesizing acyclic xanthophylls: combinatorial biosynthesis and directed evolution.
Mijts BN, Lee PC, Schmidt-Dannert C. Mijts BN, et al. Chem Biol. 2005 Apr;12(4):453-60. doi: 10.1016/j.chembiol.2005.02.010. Chem Biol. 2005. PMID: 15850982 - [Gene and gene engineering of carotenoid biosynthesis].
Tao J, Zhang SL, Xu CJ, An XM, Zhang LC. Tao J, et al. Sheng Wu Gong Cheng Xue Bao. 2002 May;18(3):276-81. Sheng Wu Gong Cheng Xue Bao. 2002. PMID: 12192856 Review. Chinese.
Cited by
- Genetically engineered biosynthetic pathways for nonnatural C60 carotenoids using C5-elongases and C50-cyclases in Escherichia coli.
Li L, Furubayashi M, Wang S, Maoka T, Kawai-Noma S, Saito K, Umeno D. Li L, et al. Sci Rep. 2019 Feb 27;9(1):2982. doi: 10.1038/s41598-019-39289-w. Sci Rep. 2019. PMID: 30814614 Free PMC article. - Genetic enhancement of Brassica napus seed quality.
Hannoufa A, Pillai BV, Chellamma S. Hannoufa A, et al. Transgenic Res. 2014 Feb;23(1):39-52. doi: 10.1007/s11248-013-9742-3. Epub 2013 Aug 27. Transgenic Res. 2014. PMID: 23979711 Review. - Seed Endophyte Microbiome of Crotalaria pumila Unpeeled: Identification of Plant-Beneficial Methylobacteria.
Sánchez-López AS, Pintelon I, Stevens V, Imperato V, Timmermans JP, González-Chávez C, Carrillo-González R, Van Hamme J, Vangronsveld J, Thijs S. Sánchez-López AS, et al. Int J Mol Sci. 2018 Jan 19;19(1):291. doi: 10.3390/ijms19010291. Int J Mol Sci. 2018. PMID: 29351192 Free PMC article. - Evolutionary Rate Heterogeneity of Primary and Secondary Metabolic Pathway Genes in Arabidopsis thaliana.
Mukherjee D, Mukherjee A, Ghosh TC. Mukherjee D, et al. Genome Biol Evol. 2015 Nov 10;8(1):17-28. doi: 10.1093/gbe/evv217. Genome Biol Evol. 2015. PMID: 26556590 Free PMC article. - 4,4'-diaponeurosporene, a C30 carotenoid, effectively activates dendritic cells via CD36 and NF-κB signaling in a ROS independent manner.
Liu H, Xu W, Chang X, Qin T, Yin Y, Yang Q. Liu H, et al. Oncotarget. 2016 Jul 5;7(27):40978-40991. doi: 10.18632/oncotarget.9800. Oncotarget. 2016. PMID: 27276712 Free PMC article.
References
- Abecassis, V., D. Pompon, and G. Truan. 2003. Producing chimeric genes by CLERY: in vitro and in vivo recombination, p. 165-173. In F. H. Arnold and G. Georgiou (ed.), Methods in molecular biology, vol. 231. Directed evolution library creation methods and protocols. Humana Press, Totowa, N.J. - PubMed
- Aharoni, A., L. C. Keizer, H. J. Bouwmeester, Z. Sun, M. Alvarez-Huerta, H. A. Verhoeven, J. Blaas, A. M. van Houwelingen, R. C. De Vos, H. van der Voet, R. C. Jansen, M. Guis, J. Mol, R. W. Davis, M. Schena, A. J. van Tunen, and A. P. O'Connell. 2000. Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell 12:647-662. - PMC - PubMed
- Albrecht, M., S. Takaichi, N. Misawa, G. Schnurr, P. Böger, and G. Sandmann. 1997. Synthesis of atypical cyclic and acyclic hydroxy carotenoids in Escherichia coli transformants. J. Biotechnol. 58:177-185. - PubMed
- Albrecht, M., S. Takaichi, S. Steiger, Z. Y. Wang, and G. Sandmann. 2000. Novel hydroxycarotenoids with improved antioxidative properties produced by gene combination in Escherichia coli. Nat. Biotechnol. 18:843-846. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources