Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival - PubMed (original) (raw)
Comparative Study
Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival
Rainer Glass et al. J Neurosci. 2005.
Abstract
Neural precursor cells contribute to adult neurogenesis and to limited attempts of brain repair after injury. Here we report that in a murine experimental glioblastoma model, endogenous neural precursors migrate from the subventricular zone toward the tumor and surround it. The association of endogenous precursors with syngenic tumor grafts was observed, after injecting red fluorescent protein-labeled G261 cells into the caudate-putamen of transgenic mice, which express green fluorescent protein under a promoter for nestin (nestin-GFP). Fourteen days after inoculation, the nestin-GFP cells surrounded the tumors in several cell layers and expressed markers of early noncommitted and committed precursors. Nestin-GFP cells were further identified by a characteristic membrane current pattern as recorded in acute brain slices. 5-bromo-2-deoxyuridine labeling and dye tracing experiments revealed that the tumor-associated precursors originated from the subventricular zone. Moreover, in cultured explants from the subventricular zone, the neural precursors showed extensive tropism for glioblastomas. Tumor-induced endogenous precursor cell accumulation decreased with age of the recipient; this correlated with increased tumor size and shorter survival times in aged mice. Coinjection of glioblastoma cells with neural precursors improved the survival time of old mice to a level similar to that in young mice. Coculture experiments showed that neural precursors suppressed the rapid increase in tumor cell number, which is characteristic of glioblastoma, and induced glioblastoma cell apoptosis. Our results indicate that tumor cells attract endogenous precursor cells; the presence of precursor cells is antitumorigenic; and this cellular interaction decreases with aging.
Figures
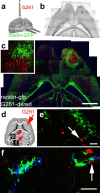
Figure 1.
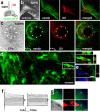
Endogenous neural precursor cells surround experimental glioblastomas. a, Illustration of the procedure and the location for grafting of G261-DsRed cells. b, Phase contrast composite image corresponding to the image in c displaying the brain morphology 14 d after tumor inoculation into a nestin-GFP mouse. c, Fluorescence microscope composite image of a horizontal section through the brain, with nestin-GFP cells (green) accumulating around a tumor (red G261 glioblastoma cells) in the right forebrain. Neural precursor cells in the contralateral hemisphere are primarily restricted to the subventricular zone. A magnified confocal image (c, inset) shows nestin-GFP-positive cells at the border zone of the glioblastoma. d, Fourteen days after tumor inoculation, some G261-DsRed cells invaded into the fimbria of the hippocampus; the arrow refers to the image in e. Other G261-DsRed cells invaded the lateral thalamus; the arrow refers to the image in f. e, Nestin-GFP-expressing precursors associate with glioblastoma cells deeply invading into the brain. f, Nestin-GFP-and doublecortin-coexpressing neural precursors track down DsRed-expressing glioblastoma cells in the thalamus. Scale bars: c, 1 mm; e, f, 40 μm.
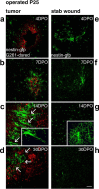
Figure 2.
Time course of the precursor cell attraction to glioblastomas. Time course and intensity of the accumulation of nestin-GFP cells at glioblastomas (a-d) or at stab wounds (e-h) are different (in mice of P25). At 4 d postoperatively (4DPO), the injection canals were still visible (a, e); later on, these were identified stereotactically. At 4DPO and 7DPO, G261-DsRed cells started to form tumors (a, b), but nestin-GFP cells accumulated similarly at tumors and controls (e, f). This changed dramatically at 14DPO. In controls of 14DPO (g) and 30DPO (h), no nestin-GFP-positive cells remained at the lesion; cells scattered throughout the lesioned hemisphere were stellate in morphology (g, inset). G261-DsRed-induced glioblastomas reached a diameter of >1 mm, closely encircled by a rim of neural precursor cells forming a layer of at least 200 μm; the tumor border is indicated by arrows in c. These nestin-GFP-positive cells typically had a bipolar morphology (c, inset). In glioblastoma-bearing mice at 30DPO, the continuous layer of nestin-GFP-positive cells around the tumor was absent, and the tumors had primarily increased in size; the tumor border is indicated by arrows in d. Scale bar, 120 μm.
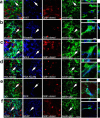
Figure 3.
Nestin-GFP-positive cells accumulating around experimental glioblastomas are characterized as genuine neural precursors. G261-DsRed cells induced glioblastomas (red) in the caudate-putamen of P25 nestin-GFP mice (nestin-GFP cells in green), which 14 d postoperatively expressed cell type-specific markers (blue). Colocalization of these markers with nestin-GFP cells is demonstrated by arrows in the micrographs and by 3D reconstructions of single cells. a, The cell cycle protein Ki67 (a marker for cell proliferation) labeled nuclei of numerous glioblastoma cells and many nestin-GFP cells. b, Musashi 1, marking neural precursors, exclusively labeled nestin-GFP-positive cells. c, NG-2, a marker for neoplastic cells, was abundant on the plasma membranes and in the cytosol of nestin-GFP-positive cells and glioblastomas. d, Staining for the neural precursor cell marker PSA-NCAM in the immediate surroundings of glioblastomas was almost exclusively detected on nestin-GFP-positive cells and was not found on glioblastoma cells. e, Immunolabeling for DCx, which controls migration in neural precursors, was only detected in the cytoplasm of nestin-GFP-positive cells in the germinal centers and around tumors. f, The intermediate filament GFAP, which is present in neural stem cells, radial glia, and astrocytes, primarily colabeled with nestin-GFP in areas close to tumors. Few cells expressing only GFAP but not nestin-GFP were observed in the area bearing G261-DsRed cells. Scale bars: overviews, 25 μm; 3D reconstructions, 6 μm.
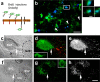
Figure 4.
Neural precursor cells from the germinative center, i.e., the subventricular zone, exhibit a strong tropism for glioblastomas. Neural precursor cells labeled preoperatively (a) appear around a glioblastoma (b). Many nestin-GFP cells at the tumor border (b, arrowheads) are labeled for BrdU (blue). Colabeling for nestin-GFP and BrdU of a single cell in b, which is highlighted by a rectangle, was confirmed by 3D reconstruction. c, An explant from the subventricular zone (SVZ) of a nestin-GFP mouse was exposed to a 3D cell aggregate of DsRed-expressing glioblastoma cells (G261); the outlines of the tumor are indicated by a dashed line. d, After 5 d of coculture, many GFP-expressing precursors have migrated into the area occupied by the tumor; these migrating neural precursors (indicated by an arrow) have a bipolar morphology. e, Single-channel micrograph indicating that neural precursors distribute exclusively into the glioblastoma. f, Two explants from the SVZ were exposed to each other and to a tissue from the cortex (Ctx; indicated by a dashed line) over a culture period of 5 d; all tissues were derived from nestin-GFP mice. g, Neural precursors from the subventricular zone stayed stationary and preserved a rounded cell morphology (single stationary cell indicated by arrows). h, Single-channel micrograph showing absence of any precursor cell migration. Scale bars: b, 20 μm; 3D reconstruction, 5 μm; h, 150 μm.
Figure 5.
Neural precursors from the subventricular zone migrate toward glioblastomas. a, Illustration of the location and procedure for injections of G261 cells and DiI. b, Five days postoperatively (5DPO), DiI is colocalized with nestin-GFP and distributed in the ventricular and subventricular zones. c, At 14DPO, a glioblastoma has developed in the caudate-putamen (CPu) of a nestin-GFP mouse. Green labeled (nestin-GFP-positive) neural precursors emanating from the subventricular zone (pointed out by dots) accumulate around the tumor. The glioblastoma (arrowheads) is encircled by DiI-positive (red fluorescent) cells. Nestin-GFP-positive cells colocalized with the DiI staining. d, At the single-cell level, DiI is almost exclusively associated with nestin-GFP cells; DiI staining is located in nestin-GFP-positive neural precursor cells (3D reconstruction). e, Triple-positive cell labeling for doublecortin, nestin-GFP, and DiI. A first population (n = 8) of DiI/nestin-GFP cells was characterized by rapidly activating and inactivating inward currents elicited with depolarization followed by outward currents. The threshold for activation of the inward currents was at approximately -40 mV. Furthermore, these cells express time-dependent hyperpolarization-activated currents (f, left). A second population (n = 6) of DiI/nestin-GFP cells expressed passive, noninactivating currents with a linear current-voltage relationship and a reversal potential of -74 mV (f, right). h, DiI**/**nestin-GFP cells for electrophysiology could be readily identified; nestin-GFP, DiI, and a merged image of an acute brain slice (phase contrast image) are shown, and correct application of the patch pipette was verified by dye filling; note that some neighboring cells are dye-coupled. Scale bars: b, c, 400 μm; d, 20 μm; 3D reconstructions, 6 μm; g, 100 μm.
Figure 6.
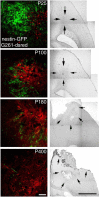
The decrease of neural precursors at glioblastomas in mice of increasing age relates to the severity of the pathology. The distribution and abundance of nestin-GFP-positive and tumor cells 14 d after inoculation was compared in animals at P25, P100, P180, and P400. The severity of the pathological state augmented with increasing age and is illustrated by representative phase contrast composite images, with arrows pointing out the extent of the main tumor mass. Glioblastomas in 100-d-old mice comprised a confined tumor mass, whereas in older mice, the G261 tumor cells invaded the brain parenchyma at P400, causing necrotic centers and local bleeding. Many nestin-GFP-positive cells surrounded the tumor in the 25-d-old animals, whereas at P100, only single, but nevertheless large, patches of neural precursor cells were found. At P180 and P400, single scattered neural precursor cells were deeply embedded within the tumors. Scale bars: fluorescence images, 50 μm; phase contrast composites, 1 mm.
Figure 7.
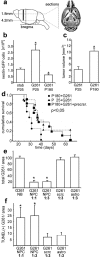
Neural precursors prolong survival of glioblastomas in vivo and are antitumorigenic in vitro. a, Stereological cell counting of serial horizontal sections was performed in the area where the main tumor mass was located. The number of nestin-GFP-positive cells within the defined area (b) was significantly (p < 0.05) larger in glioblastoma-bearing mice at P25 than in those of P180 or stab wound controls at P25 (b). c, The tumor volume in nestin-GFP mice injected with G261-DsRed cells was significantly (p < 0.05) smaller in young animals (P25) and than in old ones (P180). d, Survival curves of young (P25+G261; n = 8) and old (P180+G261; n = 9) animals after G261 cell inoculation. The young animals survived significantly longer than the older but identically treated animals. However, when old mice (P180) were injected with a mixture of cultured adult neural precursor and G261 cells (P180+G261+precrsr.; n = 10), the old animals survived better, similar to young mice. e, Glioblastoma cells (G261) were either cultured in unconditioned precursor cell medium (NB) or cocultured with neural precursors (NPC) in different ratios (1:1 or 1:3); G261 cells were also cocultured with scrc-1008 fibroblasts (scrc) and astrocytes (astro); after 72 h, the number of G261 cells detected in cocultures with NPC was five times lower than in the controls. f, After 72 h of coculturing, the number of TUNEL-positive (apoptotic) G261 cells was four times greater in cocultures with NPC than in controls.
Similar articles
- The antitumorigenic response of neural precursors depends on subventricular proliferation and age.
Walzlein JH, Synowitz M, Engels B, Markovic DS, Gabrusiewicz K, Nikolaev E, Yoshikawa K, Kaminska B, Kempermann G, Uckert W, Kaczmarek L, Kettenmann H, Glass R. Walzlein JH, et al. Stem Cells. 2008 Nov;26(11):2945-54. doi: 10.1634/stemcells.2008-0307. Epub 2008 Aug 28. Stem Cells. 2008. PMID: 18757298 - Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord.
Lepore AC, Fischer I. Lepore AC, et al. Exp Neurol. 2005 Jul;194(1):230-42. doi: 10.1016/j.expneurol.2005.02.020. Exp Neurol. 2005. PMID: 15899260 - Novel Peripherally Derived Neural-Like Stem Cells as Therapeutic Carriers for Treating Glioblastomas.
Birbrair A, Sattiraju A, Zhu D, Zulato G, Batista I, Nguyen VT, Messi ML, Solingapuram Sai KK, Marini FC, Delbono O, Mintz A. Birbrair A, et al. Stem Cells Transl Med. 2017 Feb;6(2):471-481. doi: 10.5966/sctm.2016-0007. Epub 2016 Sep 14. Stem Cells Transl Med. 2017. PMID: 28191774 Free PMC article. - Investigating the use of primary adult subventricular zone neural precursor cells for neuronal replacement therapies.
Lim DA, Flames N, Collado L, Herrera DG. Lim DA, et al. Brain Res Bull. 2002 Apr;57(6):759-64. doi: 10.1016/s0361-9230(01)00768-7. Brain Res Bull. 2002. PMID: 12031272 Review. - The subventricular zone: source of neuronal precursors for brain repair.
Alvarez-Buylla A, Herrera DG, Wichterle H. Alvarez-Buylla A, et al. Prog Brain Res. 2000;127:1-11. doi: 10.1016/s0079-6123(00)27002-7. Prog Brain Res. 2000. PMID: 11142024 Review.
Cited by
- Endogenous brain pericytes are widely activated and contribute to mouse glioma microvasculature.
Svensson A, Özen I, Genové G, Paul G, Bengzon J. Svensson A, et al. PLoS One. 2015 Apr 13;10(4):e0123553. doi: 10.1371/journal.pone.0123553. eCollection 2015. PLoS One. 2015. PMID: 25875288 Free PMC article. - Cellular host responses to gliomas.
Najbauer J, Huszthy PC, Barish ME, Garcia E, Metz MZ, Myers SM, Gutova M, Frank RT, Miletic H, Kendall SE, Glackin CA, Bjerkvig R, Aboody KS. Najbauer J, et al. PLoS One. 2012;7(4):e35150. doi: 10.1371/journal.pone.0035150. Epub 2012 Apr 23. PLoS One. 2012. PMID: 22539956 Free PMC article. - Potential of adult neural stem cells for cellular therapy.
Taupin P. Taupin P. Biologics. 2007 Mar;1(1):53-8. Biologics. 2007. PMID: 19707348 Free PMC article. - Pericyte deficiencies lead to aberrant tumor vascularizaton in the brain of the NG2 null mouse.
Huang FJ, You WK, Bonaldo P, Seyfried TN, Pasquale EB, Stallcup WB. Huang FJ, et al. Dev Biol. 2010 Aug 15;344(2):1035-46. doi: 10.1016/j.ydbio.2010.06.023. Epub 2010 Jun 27. Dev Biol. 2010. PMID: 20599895 Free PMC article. - Stem cells as vectors to deliver HSV/tk gene therapy for malignant gliomas.
Rath P, Shi H, Maruniak JA, Litofsky NS, Maria BL, Kirk MD. Rath P, et al. Curr Stem Cell Res Ther. 2009 Jan;4(1):44-9. doi: 10.2174/157488809787169138. Curr Stem Cell Res Ther. 2009. PMID: 19149629 Free PMC article. Review.
References
- Arnhold S, Hilgers M, Lenartz D, Semkova I, Kochanek S, Voges J, Andressen C, Addicks K (2003) Neural precursor cells as carriers for a gene therapeutical approach in tumor therapy. Cell Transplant 12: 827-837. - PubMed
- Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di Meco F, De Fraja C, Vescovi A, Cattaneo E, Finocchiaro G (2000) Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med 6: 447-450. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical