Agonist activation of arachidonate-regulated Ca2+-selective (ARC) channels in murine parotid and pancreatic acinar cells - PubMed (original) (raw)
Comparative Study
Agonist activation of arachidonate-regulated Ca2+-selective (ARC) channels in murine parotid and pancreatic acinar cells
Olivier Mignen et al. J Physiol. 2005.
Abstract
ARC channels (arachidonate-regulated Ca(2+)-selective channels) are a novel type of highly Ca(2+)-selective channel that are specifically activated by low concentrations of agonist-induced arachidonic acid. This activation occurs in the absence of any depletion of internal Ca(2+) stores (i.e. they are 'non-capacitative'). Previous studies in HEK293 cells have shown that these channels provide the predominant pathway for the entry of Ca(2+) seen at low agonist concentrations where oscillatory [Ca(2+)](i) signals are typically produced. In contrast, activation of the more widely studied store-operated Ca(2+) channels (e.g. CRAC channels) is only seen at higher agonist concentrations where sustained 'plateau-type'[Ca(2+)](i) responses are observed. We have now demonstrated the presence of ARC channels in both parotid and pancreatic acinar cells and shown that, again, they are specifically activated by the low concentrations of appropriate agonists (carbachol in the parotid, and both carbachol and cholecystokinin in the pancreas) that are associated with oscillatory [Ca(2+)](i) signals in these cells. Uncoupling the receptor-mediated activation of cytosolic phospholipase A(2) (cPLA(2)) with isotetrandrine reduces the activation of the ARC channels by carbachol and, correspondingly, markedly inhibits the [Ca(2+)](i) signals induced by low carbachol concentrations, whilst those signals seen at high agonist concentrations are essentially unaffected. Interestingly, in the pancreatic acinar cells, activation by cholecystokinin induces a current through the ARC channels that is only approximately 60% of that seen with carbachol. This is consistent with previous reports indicating that carbachol-induced [Ca(2+)](i) signals in these cells are much more dependent on Ca(2+) entry than are the cholecystokinin-induced responses.
Figures
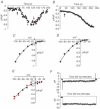
Figure 1. Arachidonic acid-activated currents in parotid and pancreatic acinar cells
A and B, representative traces showing the effect of exogenous arachidonic acid (8 μ
m
, added at black arrow) on currents measured at −80 mV in single isolated parotid acinar cells (A), and isolated pancreatic acinar cells (B). In A, La3+ (50 μ
m
) was added at the red arrow. C and D, representative current–voltage relationships of the currents activated by addition of arachidonic acid (8 μ
m
) in parotid (C) and pancreatic acinar cells (D). E, comparison of the arachidonic acid-activated currents in pancreatic acinar cells measured in normal external solution (black symbols), and that measured in a solution in which external Na+ was replaced with the impermeant cation NMDG+ (red symbols). F, representative currents recorded during 250 ms pulses to −80 mV from a holding potential of 0 mV in isolated parotid acinar cells (top trace) and pancreatic acinar cells (lower trace) following activation with arachidonic acid (8 μ
m
). Capacity transients were corrected as described in Methods.
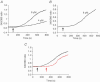
Figure 2. Effect of arachidonic acid on [Ca2+]i in parotid and pancreatic acinar cells
A and B, representative traces showing the effect of adding arachidonic acid at the indicated concentrations (black arrow) on [Ca2+]i measured as the fluorescence ratio (_F_340/380) of intracellularly loaded fura-2 in isolated parotid acinar cells (A), and isolated pancreatic acinar cells (B). C, inhibition of the arachidonic acid-induced increase in [Ca2+]i in parotid cells by La3+. Arachidonic acid (8 μ
m
) was added (black arrow) either in the absence (black trace) or presence (red trace) of 50 μ
m
La3+. The La3+ was subsequently removed (red arrow).
Figure 3. Activation of ARC channels by low concentrations of carbachol in parotid acinar cells
A, representative trace showing the effect of carbachol (250 n
m
, added at arrow) on currents measured at −80 mV in single isolated parotid acinar cells. B, representative current–voltage relationship of the current activated by 250 n
m
carbachol in parotid acinar cells. C, representative current–voltage relationships of the currents activated by 250 n
m
carbachol in parotid acinar cells in the presence (red trace) and absence (black trace) of isotetrandrine (10 μ
m
). D, representative current recorded during a 250 ms pulse to −80 mV from a holding potential of 0 mV in an isolated parotid acinar cell following activation with 250 n
m
carbachol measured in the presence of 10 μ
m
isotetrandrine. Capacity transients were corrected as described in Methods.
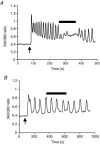
Figure 4. Effect of isotetrandrine on the [Ca2+]i signals activated by low and high carbachol concentrations in isolated parotid acinar cells
[Ca2+]i was measured as the _F_340/380 ratio of intracellularly loaded fura-2 as described. At the first arrow, carbachol (300 n
m
) was added; at the second arrow the carbachol concentration was increased to 10 μ
m
. The black bars indicate when isotetrandrine (10 μ
m
) was present.
Figure 5. Activation of ARC channels by carbachol and cholecystokinin in pancreatic acinar cells
A and B, representative traces showing the effect of carbachol (200 n
m
) and CCK-8 (20 p
m
), respectively, on currents measured at −80 mV in single isolated pancreatic acinar cells. The agonists were added at the arrow in each case. C, mean (±
s.e.m.
) current–voltage relationships of the currents activated by carbachol (200 n
m
, black circles), and CCK-8 (20 p
m
, red circles) in isolated pancreatic acinar cells. D, representative currents recorded during 250 ms pulses to −80 mV from a holding potential of 0 mV in isolated pancreatic acinar cells in the presence of carbachol (200 n
m
, top trace) and CCK-8 (20 p
m
, lower trace). Capacity transients were corrected as described in Methods.
Figure 6. Effect of isotetrandrine on the [Ca2+]i signals activated by carbachol and cholecystokinin in pancreatic acinar cells
Changes in [Ca2+]i in isolated pancreatic acinar cells, measured as _F_340/380 of intracellularly loaded fura-2, following addition of 200 n
m
carbachol (A), or 20 p
m
CCK-8 (B). In each case, the agonists were added at the arrow. Subsequently, isotetrandrine (10 μ
m
) was added as indicated by the black bar. Notice the different time scales of the responses.
Similar articles
- ARC channels: a novel pathway for receptor-activated calcium entry.
Shuttleworth TJ, Thompson JL, Mignen O. Shuttleworth TJ, et al. Physiology (Bethesda). 2004 Dec;19:355-61. doi: 10.1152/physiol.00018.2004. Physiology (Bethesda). 2004. PMID: 15546853 Review. - Orai channel-dependent activation of phospholipase C-δ: a novel mechanism for the effects of calcium entry on calcium oscillations.
Thompson JL, Shuttleworth TJ. Thompson JL, et al. J Physiol. 2011 Nov 1;589(Pt 21):5057-69. doi: 10.1113/jphysiol.2011.214437. Epub 2011 Aug 30. J Physiol. 2011. PMID: 21878525 Free PMC article. - Ca2+ selectivity and fatty acid specificity of the noncapacitative, arachidonate-regulated Ca2+ (ARC) channels.
Mignen O, Thompson JL, Shuttleworth TJ. Mignen O, et al. J Biol Chem. 2003 Mar 21;278(12):10174-81. doi: 10.1074/jbc.M212536200. Epub 2003 Jan 9. J Biol Chem. 2003. PMID: 12522216 - I(ARC), a novel arachidonate-regulated, noncapacitative Ca(2+) entry channel.
Mignen O, Shuttleworth TJ. Mignen O, et al. J Biol Chem. 2000 Mar 31;275(13):9114-9. doi: 10.1074/jbc.275.13.9114. J Biol Chem. 2000. PMID: 10734044 - Calcium entry and the control of calcium oscillations.
Shuttleworth TJ, Mignen O. Shuttleworth TJ, et al. Biochem Soc Trans. 2003 Oct;31(Pt 5):916-9. doi: 10.1042/bst0310916. Biochem Soc Trans. 2003. PMID: 14505448 Review.
Cited by
- Arachidonate-regulated Ca2+-selective (ARC) channel activity is modulated by phosphorylation and involves an A-kinase anchoring protein.
Mignen O, Thompson JL, Shuttleworth TJ. Mignen O, et al. J Physiol. 2005 Sep 15;567(Pt 3):787-98. doi: 10.1113/jphysiol.2005.090209. Epub 2005 Jun 30. J Physiol. 2005. PMID: 15994185 Free PMC article. - Arachidonate-regulated Ca(2+) influx in human airway smooth muscle.
Thompson MA, Prakash YS, Pabelick CM. Thompson MA, et al. Am J Respir Cell Mol Biol. 2014 Jul;51(1):68-76. doi: 10.1165/rcmb.2013-0144OC. Am J Respir Cell Mol Biol. 2014. PMID: 24471656 Free PMC article. - Emerging roles of Orai3 in pathophysiology.
Motiani RK, Stolwijk JA, Newton RL, Zhang X, Trebak M. Motiani RK, et al. Channels (Austin). 2013 Sep-Oct;7(5):392-401. doi: 10.4161/chan.24960. Epub 2013 May 21. Channels (Austin). 2013. PMID: 23695829 Free PMC article. Review. - Modelling the transition from simple to complex Ca²⁺ oscillations in pancreatic acinar cells.
Manhas N, Sneyd J, Pardasani KR. Manhas N, et al. J Biosci. 2014 Jun;39(3):463-84. doi: 10.1007/s12038-014-9430-3. J Biosci. 2014. PMID: 24845510 - Modelling mechanism of calcium oscillations in pancreatic acinar cells.
Manhas N, Pardasani KR. Manhas N, et al. J Bioenerg Biomembr. 2014 Oct;46(5):403-20. doi: 10.1007/s10863-014-9561-0. Epub 2014 Jul 11. J Bioenerg Biomembr. 2014. PMID: 25011561
References
- Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, Melvin JE. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem. 2004;279:47681–47687. - PubMed
- Bers DM, Patton CW, Nuccitelli R. A practical guide to the preparation of Ca2+ buffers. Meths Cell Biol. 1994;40:1–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK56468/DK/NIDDK NIH HHS/United States
- DE13539/DE/NIDCR NIH HHS/United States
- P01 DE013539/DE/NIDCR NIH HHS/United States
- GM40457/GM/NIGMS NIH HHS/United States
- R01 GM040457/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous