PTEN as an effector in the signaling of antimigratory G protein-coupled receptor - PubMed (original) (raw)
PTEN as an effector in the signaling of antimigratory G protein-coupled receptor
Teresa Sanchez et al. Proc Natl Acad Sci U S A. 2005.
Abstract
PTEN, a tumor suppressor phosphatase, is important in the regulation of cell migration and invasion. Physiological regulation of PTEN (phosphatase and tensin homolog deleted on chromosome 10) by cell surface receptors has not been described. Here, we show that the bioactive lipid sphingosine 1-phosphate (S1P), which acts through the S1P2 receptor (S1P2R) G protein-coupled receptor (GPCR) to inhibit cell migration, utilizes PTEN as a signaling intermediate. S1P2R inhibition of cell migration is abrogated by dominant-negative PTEN expression. S1P was unable to efficiently inhibit the migration of Pten(DeltaloxP/DeltaloxP) mouse embryonic fibroblasts; however, the antimigratory effect was restored upon the expression of PTEN. S1P2R activation of Rho GTPase is not affected in Pten(DeltaloxP/DeltaloxP) cells, and dominant-negative Rho GTPase reversed S1P inhibition of cell migration in WT cells but not in Pten(DeltaloxP/DeltaloxP) cells, suggesting that PTEN acts downstream of the Rho GTPase. Ligand activation of the S1P2R receptor stimulated the coimmunoprecipitation of S1P2R and PTEN. Interestingly, S1P2R signaling increased PTEN phosphatase activity in membrane fractions. Furthermore, tyrosine phosphorylation of PTEN was stimulated by S1P2R signaling. These data suggest that the S1P2R receptor actively regulates the PTEN phosphatase by a Rho GTPase-dependent pathway to inhibit cell migration. GPCR regulation of PTEN maybe a general mechanism in signaling events of cell migration and invasion.
Figures
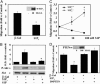
Fig. 1.
S1P2R inhibits migration in HUVECs and MEFs. (A) S1P-induced migration in adenovirus control (β-gal) and S1P2R-transduced (S1P2) endothelial cells. HUVECs were treated with 100 nM S1P or were left untreated, and cell migration was quantified as described. (Inset) S1P2R polypeptide expression in S1P2R virus-transduced HUVECs was determined by immunoprecipitation with anti-Flag antibody followed by immunoblotting with the same antibody, as described in Materials and Methods. *, P < 0.01 vs. β-gal-transduced HUVECs. (B) Phospho-Akt and total Akt levels in adenovirus control (β-gal) and S1P2R-transduced (S1P2) endothelial cells stimulated with 100 nM S1P for 10 min. A representative blot of three is shown. Data are the mean ± SE; n = 3. *, P < 0.01 vs. β-gal-transduced HUVECs. (C) S1P-induced migration of MEFs derived from WT (S1P2+/+) or S1P2R null (S1P2–/–) mice. *, P < 0.01, WT vs. S1P2R null. (D) S1P-induced migration of endothelial cells infected with control (β-gal), S1P2R, or dnPTEN adenoviruses. Cells that were not infected with dnPTEN received the same dose of adenovirus control. Data are the mean ± SE of triplicates from a representative experiment; n = 2–4. *, P < 0.01 vs. control virus-infected cells. (Inset) PTEN levels after infection with 100 multiplicity of infection of dnPTEN virus.
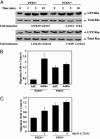
Fig. 2.
PTEN mediates S1P2R-dependent inhibition of migration in MEFs. (A) Migration of Pten ΔloxP/ΔloxP (PTEN–/–) and Pten+/+ MEF cells toward S1P. (Inset) PTEN expression in Pten ΔloxP/ΔloxP and WT MEFs. *, P < 0.01 vs. Pten+/+ MEFs. (B) Migration of Pten ΔloxP/ΔloxP (PTEN–/–) and WT MEFs toward FTY720-P. (C) Migration of adenovirus control-transduced Pten ΔloxP/ΔloxP MEFs (PTEN–/– + AdC) and Pten+/+ MEFs (Pten+/+ + AdC) and WT PTEN adenovirus-transduced Pten ΔloxP/ΔloxP MEFs (PTEN–/– + Ad WT PTEN) toward different concentrations of S1P. (Inset) PTEN expression in Pten ΔloxP/ΔloxP and Pten+/+ MEFs infected with the different adenoviruses. Fold induction vs. vehicle control is represented. Data represent the mean ± SE of triplicate values of a representative experiment; n = 2–4. (D) Phospho-Akt and total Akt levels in Pten ΔloxP/ΔloxP (PTEN–/– and Pten+/+ MEFs stimulated for 10 min with vehicle control (C), 100 nM S1P (S1P), and 10 nM FTY720-P (Fp). A representative blot of three is shown. Values are the mean ± SE; n = 3.
Fig. 3.
Rho, p160-ROCK, and PTEN mediate S1P2R antimigratory action. (A) Levels of active Rho (GTP-Rho), total Rho, active Rac (GTP-Rac), and total Rac in Pten ΔloxP/ΔloxP (PTEN–/–) and WT (PTEN+/+) MEFs after 2-, 5-, and 10-min S1P stimulation. A representative blot of four is shown. Values are the mean ± SE of the fold induction; n = 4. (B) Migration of control adenovirus (AdC) and dominant-negative Rho adenovirus (dnRho)-transduced Pten+/+ (PTEN+/+) and Pten ΔloxP/ΔloxP (PTEN–/–) MEF cells toward 10 nM S1P. *, P < 0.01 vs. adenovirus control-transduced cells. (C) Pten+/+ (PTEN+/+) and Pten ΔloxP/ΔloxP (PTEN–/–) MEFs were pretreated with vehicle control or 10 μM Y-27632 for 30 min. Then, a migration experiment toward 10 nM S1P was performed as described. These treatments were also present at the upper and lower chamber during the migration experiment. *, P < 0.01 vs. nontreated cells. Fold induction vs. vehicle control (basal motility in the absence of S1P) is plotted. Data are the mean ± SE of triplicates from one representative experiment; n = 2–3.
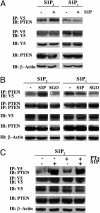
Fig. 4.
Ligand-dependent association of S1P2R and PTEN. (A) HEK293T cells were transfected with HA-PTEN and nV5-S1P2R (S1P2) or HA-PTEN and V5-S1P1R (S1P1). Twenty-four hours after transfection, cells were serum-starved and stimulated with vehicle control (–) or 100 nM S1P (+) for 10 min. Cell lysis and immunoprecipitation were performed. (B) Cells were treated with vehicle control (–), 100 nM S1P (S1P), or 100 nM sphingosine (SGO) for 10 min. (C) PTEN-S1P2R association is not inhibited by PTx treatment. HEK293T cells were transfected with HA-PTEN and V5-S1P2R. During serum starvation, cells were treated with 200 ng/ml PTx where indicated. They were then stimulated with 100 nM S1P (S1P) for 10 min. Cell lysis and immunoprecipitation were performed as indicated above. A representative experiment of three is shown.
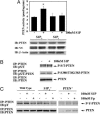
Fig. 5.
S1P stimulation triggers an increase in PTEN-specific activity and tyrosine phosphorylation. (A) S1P stimulation triggers an increase in PTEN-specific activity in HA-PTEN and S1P2R HEK293T cells. Cells were transfected with HA-PTEN and nV5-S1P2R (S1P2) or HA-PTEN and nV5-S1P1R (S1P1). After serum starvation, cells were stimulated for 5 min with vehicle control (–) or 100 nM S1P (+). Membrane fraction was isolated, and phosphatase activity toward phosphatidylinositol-3,4,5-triphosphate present in lipid vesicles was measured from immunoprecipitated PTEN from membrane fraction as described in Materials and Methods. Data represent the mean ± SE of quadruplicates from a representative experiment; n = 2. *, P < 0.05 vs. vehicle control. (B) S1P stimulation did not induce changes in Thr/Ser-phosphorylated PTEN. MEFs were serum-starved and stimulated with S1P or vehicle control for 15 min. Cell extract (1 mg) was immunoprecipitated with PTEN antibody, followed by immunoblotting with anti-phospho-serine and -threonine general antibody (P-S/T-PTEN), anti-phospho-Ser-380/Thr-382 and 383-PTEN antibody (P-S380/T382/383-PTEN), or PTEN antibody. (C) S1P stimulation induced tyrosine phosphorylation of PTEN. MEFs from WT, S1P2R null (S1P2–/–), or Pten null (PTEN–/–) mice were serum-starved, preincubated with 30 μM pervanadate for 15 min, and stimulated with S1P, FTY720-P (Fp), or vehicle control for another 15 min. Cells were lysed, and 1 mg of cell extract was immunoprecipitated with PTEN antibody, followed by immunoblotting with anti-phospho-tyrosine antibody (pY) or PTEN antibody. A representative blot is shown; n = 2–4.
Similar articles
- Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN.
Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Sanchez T, et al. Arterioscler Thromb Vasc Biol. 2007 Jun;27(6):1312-8. doi: 10.1161/ATVBAHA.107.143735. Epub 2007 Apr 12. Arterioscler Thromb Vasc Biol. 2007. PMID: 17431187 - The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration.
Lepley D, Paik JH, Hla T, Ferrer F. Lepley D, et al. Cancer Res. 2005 May 1;65(9):3788-95. doi: 10.1158/0008-5472.CAN-04-2311. Cancer Res. 2005. PMID: 15867375 - Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades.
Lu Y, Yu Q, Liu JH, Zhang J, Wang H, Koul D, McMurray JS, Fang X, Yung WK, Siminovitch KA, Mills GB. Lu Y, et al. J Biol Chem. 2003 Oct 10;278(41):40057-66. doi: 10.1074/jbc.M303621200. Epub 2003 Jul 17. J Biol Chem. 2003. PMID: 12869565 - PTEN and myotubularin phosphatases: from 3-phosphoinositide dephosphorylation to disease.
Wishart MJ, Dixon JE. Wishart MJ, et al. Trends Cell Biol. 2002 Dec;12(12):579-85. doi: 10.1016/s0962-8924(02)02412-1. Trends Cell Biol. 2002. PMID: 12495846 Review. - Pten signaling in gliomas.
Knobbe CB, Merlo A, Reifenberger G. Knobbe CB, et al. Neuro Oncol. 2002 Jul;4(3):196-211. Neuro Oncol. 2002. PMID: 12084351 Free PMC article. Review.
Cited by
- Critical role of sphingosine-1-phosphate receptor 2 (S1PR2) in acute vascular inflammation.
Zhang G, Yang L, Kim GS, Ryan K, Lu S, O'Donnell RK, Spokes K, Shapiro N, Aird WC, Kluk MJ, Yano K, Sanchez T. Zhang G, et al. Blood. 2013 Jul 18;122(3):443-55. doi: 10.1182/blood-2012-11-467191. Epub 2013 May 30. Blood. 2013. PMID: 23723450 Free PMC article. - The emerging role of FTY720 (Fingolimod) in cancer treatment.
White C, Alshaker H, Cooper C, Winkler M, Pchejetski D. White C, et al. Oncotarget. 2016 Apr 26;7(17):23106-27. doi: 10.18632/oncotarget.7145. Oncotarget. 2016. PMID: 27036015 Free PMC article. Review. - Targeting sphingosine-1-phosphate for cancer therapy.
Sabbadini RA. Sabbadini RA. Br J Cancer. 2006 Nov 6;95(9):1131-5. doi: 10.1038/sj.bjc.6603400. Epub 2006 Oct 3. Br J Cancer. 2006. PMID: 17024123 Free PMC article. Review. - Bioactive lysolipids in cancer and angiogenesis.
Hisano Y, Hla T. Hisano Y, et al. Pharmacol Ther. 2019 Jan;193:91-98. doi: 10.1016/j.pharmthera.2018.07.006. Epub 2018 Jul 23. Pharmacol Ther. 2019. PMID: 30048709 Free PMC article. Review. - Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature.
Lee JF, Gordon S, Estrada R, Wang L, Siow DL, Wattenberg BW, Lominadze D, Lee MJ. Lee JF, et al. Am J Physiol Heart Circ Physiol. 2009 Jan;296(1):H33-42. doi: 10.1152/ajpheart.00097.2008. Epub 2008 Nov 14. Am J Physiol Heart Circ Physiol. 2009. PMID: 19011048 Free PMC article.
References
- Maehama, T. & Dixon, J. E. (1998) J. Biol. Chem. 273, 13375–13378. - PubMed
- Tamura, M., Gu, J., Matsumoto, K., Aota, S., Parsons, R. & Yamada, K. M. (1998) Science 280, 1614–1617. - PubMed
- Li, J., Yen, C., Liaw, D., Podsypanina, K., Bose, S., Wang, S. I., Puc, J., Miliaresis, C., Rodgers, L., McCombie, R., et al. (1997) Science 275, 1943–1947. - PubMed
- Liaw, D., Marsh, D. J., Li, J., Dahia, P. L., Wang, S. I., Zheng, Z., Bose, S., Call, K. M., Tsou, H. C., Peacocke, M., et al. (1997) Nat. Genet. 16, 64–67. - PubMed
- Steck, P. A., Pershouse, M. A., Jasser, S. A., Yung, W. K., Lin, H., Ligon, A. H., Langford, L. A., Baumgard, M. L., Hattier, T., Davis, T., et al. (1997) Nat. Genet. 15, 356–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL70694/HL/NHLBI NIH HHS/United States
- P01 HL070694/HL/NHLBI NIH HHS/United States
- R37 HL067330/HL/NHLBI NIH HHS/United States
- R01 HL067330/HL/NHLBI NIH HHS/United States
- HL67330/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials