Generation of 5-(2'-deoxycytidyl)methyl radical and the formation of intrastrand cross-link lesions in oligodeoxyribonucleotides - PubMed (original) (raw)
Generation of 5-(2'-deoxycytidyl)methyl radical and the formation of intrastrand cross-link lesions in oligodeoxyribonucleotides
Qibin Zhang et al. Nucleic Acids Res. 2005.
Abstract
Hydroxyl radical is one of the major reactive oxygen species (ROS) formed from gamma-radiolysis of water or Fenton reaction, and it can abstract one hydrogen atom from the methyl carbon atom of thymine and 5-methylcytosine to give the 5-methyl radical of the pyrimidine bases. The latter radical can also be induced from Type-I photo-oxidation process. Here, we examined the reactivity of the independently generated 5-(2'-deoxycytidyl)methyl radical (I) in single- and double-stranded oligodeoxyribonucleotides (ODNs). It was found that an intrastrand cross-link lesion, in which the methyl carbon atom of 5-methylcytosine and the C8 carbon atom of guanine are covalently bonded, could be formed from the independently generated radical at both GmC and mCG sites, with the yield being much higher at the former site. We also showed by LC-MS/MS that the same cross-link lesions were formed in mC-containing duplex ODNs upon gamma irradiation under both aerobic and anaerobic conditions, and the yield was approximately 10-fold higher under the latter conditions. The independently generated radical allows for the availability of pure, sufficient and well-characterized intrastrand cross-link lesion-bearing ODN substrates for future biochemical and biophysical characterizations. This was also the first demonstration that the coupling of radical I with its 5' neighboring guanine can occur in the presence of molecular oxygen, suggesting that the formation of this and other types of intrastrand cross-link lesions might have important implications in the cytotoxic effects of ROS.
Figures
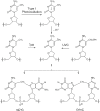
Scheme 1
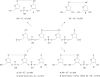
Formation of radical I and its coupling with neighboring guanine.
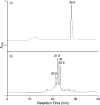
Figure 1
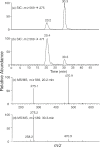
HPLC traces for the separations of single-stranded d(ACGTGYCGTGAT), where Y is the radical precursor, before (a) and after (b) 254 nm irradiation under anaerobic conditions. ‘XL’ (the 22.8 min fraction) represents d(ACGTG∧ mCCGTGAT).
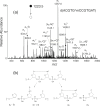
Figure 2
Product-ion spectrum of the electrospray-produced [M−3H]3− ion (m/z 1223.5) of d(ACGTG∧ mCCGTGAT) (the 22.8 min fraction in Figure 1b).
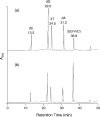
Figure 3
HPLC traces for the separation of nuclease P1 and alkaline phosphatase digestion mixture of d(ACGTG∧ mCCGTGAT) (the 22.8 min fraction in Figure 1b): (a) injection of the digestion mixture; (b) co-injection of digestion mixture and 4.5 nmol d(G∧ mC) standard.
Figure 4
LC-MS/MS results for the injection of d(mC∧ G) and d(G∧ mC) (400 fmol each). Shown are SICs for the m/z 569→275 (a) and m/z 569→471 transitions (b); product-ion spectra of the ions of m/z 569 for the fractions eluting at 20.2 min [(c), corresponding to d(mC∧ G)] and 30.3 min [(d), corresponding to d(G∧ mC)].
Scheme 2
Radical I can couple with both molecular oxygen and adjacent guanine.
Scheme 3
Possible enzymic digestion products and the m/z values of their [M + H]+ ions.
Figure 5
LC-MS/MS for the detection of cross-link lesion from the γ irradiation of self-complementary duplex ODN d(mCG)7. The irradiation mixture was digested by nuclease P1, CIP, and phosphodiesterases I and II before LC-MS analysis, and the mass spectrometer was set to monitor the fragmentation of the ion of m/z 569, which is the [M+H]+ ions of the cross-link lesion d(G∧ mC) and d(mC∧ G). Plotted are the SICs for the m/z 569→275 transition with the injections of the digestion mixtures of (a) 1 nmol ODN irradiated with γ-rays in the presence of saturated O2; (b) 1 nmol ODN irradiated with γ-rays in the presence of air; and (c) 0.5 nmol ODN irradiated with γ-rays under anaerobic conditions.
Similar articles
- The reactivity of the 5-hydroxy-5,6-dihydrothymidin-6-yl radical in oligodeoxyribonucleotides.
Zhang Q, Wang Y. Zhang Q, et al. Chem Res Toxicol. 2005 Dec;18(12):1897-906. doi: 10.1021/tx050195u. Chem Res Toxicol. 2005. PMID: 16359180 - Quantification of oxidative single-base and intrastrand cross-link lesions in unmethylated and CpG-methylated DNA induced by Fenton-type reagents.
Cao H, Wang Y. Cao H, et al. Nucleic Acids Res. 2007;35(14):4833-44. doi: 10.1093/nar/gkm497. Epub 2007 Jul 10. Nucleic Acids Res. 2007. PMID: 17626047 Free PMC article. - Free radical-induced double lesions in DNA.
Box HC, Dawidzik JB, Budzinski EE. Box HC, et al. Free Radic Biol Med. 2001 Oct 1;31(7):856-68. doi: 10.1016/s0891-5849(01)00653-0. Free Radic Biol Med. 2001. PMID: 11585704 Review. - [Free oxygen radiacals and kidney diseases--part I].
Sakac V, Sakac M. Sakac V, et al. Med Pregl. 2000 Sep-Oct;53(9-10):463-74. Med Pregl. 2000. PMID: 11320727 Review. Croatian.
Cited by
- DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene.
Besaratinia A, Synold TW, Chen HH, Chang C, Xi B, Riggs AD, Pfeifer GP. Besaratinia A, et al. Proc Natl Acad Sci U S A. 2005 Jul 19;102(29):10058-63. doi: 10.1073/pnas.0502311102. Epub 2005 Jul 11. Proc Natl Acad Sci U S A. 2005. PMID: 16009942 Free PMC article. - Occurrence, Biological Consequences, and Human Health Relevance of Oxidative Stress-Induced DNA Damage.
Yu Y, Cui Y, Niedernhofer LJ, Wang Y. Yu Y, et al. Chem Res Toxicol. 2016 Dec 19;29(12):2008-2039. doi: 10.1021/acs.chemrestox.6b00265. Epub 2016 Nov 7. Chem Res Toxicol. 2016. PMID: 27989142 Free PMC article. Review. - The mutagenicity of thymidine glycol in Escherichia coli is increased when it is part of a tandem lesion.
Huang H, Imoto S, Greenberg MM. Huang H, et al. Biochemistry. 2009 Aug 25;48(33):7833-41. doi: 10.1021/bi900927d. Biochemistry. 2009. PMID: 19618962 Free PMC article. - Sequence-dependent formation of intrastrand crosslink products from the UVB irradiation of duplex DNA containing a 5-bromo-2'-deoxyuridine or 5-bromo-2'-deoxycytidine.
Zeng Y, Wang Y. Zeng Y, et al. Nucleic Acids Res. 2006;34(22):6521-9. doi: 10.1093/nar/gkl892. Epub 2006 Nov 27. Nucleic Acids Res. 2006. PMID: 17130170 Free PMC article. - One-electron oxidation reactions of purine and pyrimidine bases in cellular DNA.
Cadet J, Wagner JR, Shafirovich V, Geacintov NE. Cadet J, et al. Int J Radiat Biol. 2014 Jun;90(6):423-32. doi: 10.3109/09553002.2013.877176. Epub 2014 Apr 3. Int J Radiat Biol. 2014. PMID: 24369822 Free PMC article. Review.
References
- Pfeifer G.P. p53 mutational spectra and the role of methylated CpG sequences. Mutat. Res. 2000;450:155–166. - PubMed
- Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. - PubMed
- Rolig R.L., McKinnon P.J. Linking DNA damage and neurodegeneration. Trends Neurosci. 2000;23:417–424. - PubMed
- Halliwell B., Gutteridge J.M.C. Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett. 1992;307:108–112. - PubMed