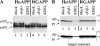beta-site specific intrabodies to decrease and prevent generation of Alzheimer's Abeta peptide - PubMed (original) (raw)
beta-site specific intrabodies to decrease and prevent generation of Alzheimer's Abeta peptide
Paolo Paganetti et al. J Cell Biol. 2005.
Abstract
Endoproteolysis of the beta-amyloid precursor protein (APP) by beta- and gamma-secretases generates the toxic amyloid beta-peptide (Abeta), which accumulates in the brain of Alzheimer's disease (AD) patients. Here, we established a novel approach to regulate production of Abeta based on intracellular expression of single chain antibodies (intrabodies) raised to an epitope adjacent to the beta-secretase cleavage site of human APP. The intrabodies rapidly associated, within the endoplasmic reticulum (ER), with newly synthesized APP. One intrabody remained associated during APP transport along the secretory line, shielded the beta-secretase cleavage site and facilitated the alternative, innocuous cleavage operated by alpha-secretase. Another killer intrabody with an ER retention sequence triggered APP disposal from the ER. The first intrabody drastically inhibited and the second almost abolished generation of Abeta. Intrabodies association with specific substrates rather than with enzymes, may modulate intracellular processes linked to disease with highest specificity and may become instrumental to investigate molecular mechanisms of cellular events.
Figures
Figure 1.
Scheme of APP processing by the secretases. APP is a type I transmembrane protein with a single hydrophobic domain for membrane retention. The amyloidogenic processing of APP produces the β-amyloid peptide (Aβ) through sequential cleavages by BACE at the β-site and by γ-secretase. Shedding of the APP ectodomain occurs through redundant proteolytic events at the cell surface (α-cut) or in endosomes (β-cut) by the secretases. The Swedish mutation at the β-site strongly favors BACE cleavage of APP on route to the cell surface. The 40 aa sequence of Aβ is also depicted (bold letters) as well as the 3 aa exchanged in murine Aβ (normal fonts). The EFRH epitope of the β1 antibody is mutated to EFGH in RGAPP.
Figure 2.
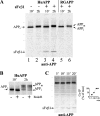
Specific binding of sFvβ1 to human APP in cells. (A) HEK cells were transfected for expression of huAPP (lanes 1 and 2), sFvβ1 and huAPP (lanes 3 and 4), or sFvβ1 and RGAPP (lanes 5 and 6). After metabolic labeling with 35S-amino acids and chasing with unlabeled amino acids, huAPP and RGAPP were immunoprecipitated from cell extracts with a carboxy-terminal APP antibody. When present, sFvβ1 associates and coprecipitates with HuAPP but not with RGAPP. APPi denotes the immature and APPm denotes the mature form of APP. (B) Analysis on 8% SDS PAGE better visualizes APP maturation and EndoH sensitivity. At 10 min labeled APPi is still EndoH sensitive; but after 2 h APP is released from the ER and APPm becomes EndoH resistant and shows increased Mr upon _N_-glycan modification, tyrosine-sulfation, and addition of _O_-glycans. (C) Kinetics of APP:sFvβ1 association were determined by coimmunoprecipitations and plotted as a function of the maximal amount of sFvβ1 coprecipitated with APP. The position of Mr markers of 200, 116, 97, 66, 45, and 32 kD is shown with thin lines.
Figure 3.
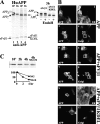
Intracellular localization and fate of huAPP coexpressed with sFvβ1 or with sFvβ1-KDEL. (A) Cells expressed huAPP and sFvβ1 or sFvβ1-KDEL. Both intrabodies associates and coprecipitates with huAPP using a carboxy-terminal antibody to APP. In the presence of sFvβ1, APPm at the end of the 3-h chase has higher Mr than newly synthesized APPi (10 min). In contrast, sFvβ1-KDEL impairs maturation of APP, no Mr shift is observed and APPi remains EndoH sensitive. (B) Indirect immunofluorescence was performed with an antibody to APP (transfected cells in panels 1, 3, 5, 7, 9, and 11) and to Cnx to visualize the ER (all cells in panels 2, 6, and 10) or Giantin to visualize the Golgi (panels 4, 8, and 12). In the presence of sFvβ1-KDEL (panels 9–12), APP and Cnx colocalizes. In sFvβ1 (panels 5–8) or mock-transfected cells (panels 1–4), APP is detected in the ER and in the Golgi. (C) Immature APP retained in the ER by sFvβ1-KDEL is degraded with a t 1/2 of ∼2 h; APP disposal is partially inhibited by the proteasomal inhibitor MG132.
Figure 4.
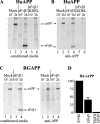
Consequences of sFvβ1 and of sFvβ1-KDEL expression on APP processing and release in the extracellular medium of the APP ectodomain. (A) 15 μl of conditioned medium (total was 1.5 ml) were boiled in sample buffer and analyzed in SDS-PAGE for determination of radiolabeled APP-ectodomain (sAPP) release from cells at 2 h (lane 2). Coexpression of sFvβ1 reduces sAPP release from cells (lane 4) and sFvβ1-KDEL virtually abolishes release of the APP ectodomain (lane 6). (B) The same samples analyzed in A were immunoprecipitated with an APP-specific antibody to demonstrate sAPP:sFvβ1 association. Only a fraction of secreted sFvβ1 coprecipitates with sAPP (compare the relative ratio sAPP vs. sFvβ1 in A and B, lanes 4). (C) Coexpressing of sFvβ1 does not lower secretion of the RGAPP ectodomain (lanes 2 and 4, cond. media; lanes 2 and 4, anti-APP) and sFvβ1 does not associate with RGAPP as shown by lack of coprecipitation (lane 4, anti-APP). (D) sAPP secretion was quantified in a series of five independent experiments. Error bar represents SD.
Figure 5.
Consequences of sFvβ1 and of sFvβ1-KDEL expression on production of Aβ and P3. (A) Western blot analysis of cell lysates with a carboxy-terminal APP-antibody visualizes immature (APPi) and mature (APPm) full-length APP at steady state. Maturation of HuAPP and RGAPP is not affected by sFvβ1 when compared with mock conditions. On the other hand, sFvβ1-KDEL fully retains HuAPP in the APPi form, but shows much lower affinity for the mutated EFGH epitope of RGAPP. (B) Aβ and P3 were identified according to their electrophoretic mobility using synthetic peptides after immunoprecipitation and Western blot analysis using two carboxy-terminal Aβ-specific antibodies. Secretase-mediated endoproteolysis of APPSwedish mainly results in production of Aβ (lane 1). Association of sFvβ1 close to the β-secretase cleavage site substantially reduces production of Aβ (lane 2), whereas sFvβ1-KDEL virtually abolishes production of Aβ (lane 3). The intrabodies have no effect on Aβ generation when the RG mutant of APP is expressed (lanes 4–6). Overexposition of gel (bottom) better visualizes the metabolite P3, and shows that sFvβ1 lowers Aβ, whereas favoring α-secretase–mediated cleavage resulting in the innocuous P3 peptide (lane 2).
Similar articles
- ELISA analysis of beta-secretase cleavage of the Swedish amyloid precursor protein in the secretory and endocytic pathways.
Steinhilb ML, Turner RS, Gaut JR. Steinhilb ML, et al. J Neurochem. 2002 Mar;80(6):1019-28. doi: 10.1046/j.0022-3042.2002.00764.x. J Neurochem. 2002. PMID: 11953452 - A distinct ER/IC gamma-secretase competes with the proteasome for cleavage of APP.
Skovronsky DM, Pijak DS, Doms RW, Lee VM. Skovronsky DM, et al. Biochemistry. 2000 Feb 1;39(4):810-7. doi: 10.1021/bi991728z. Biochemistry. 2000. PMID: 10651647 - Novel alpha-secretase cleavage of Alzheimer's amyloid beta precursor protein in the endoplasmic reticulum of COS7 cells.
Shin RW, Saido TC, Maeda M, Kitamoto T. Shin RW, et al. Neurosci Lett. 2005 Mar 7;376(1):14-9. doi: 10.1016/j.neulet.2004.11.032. Epub 2004 Dec 8. Neurosci Lett. 2005. PMID: 15694266 - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review. - The proteins BACE1 and BACE2 and beta-secretase activity in normal and Alzheimer's disease brain.
Stockley JH, O'Neill C. Stockley JH, et al. Biochem Soc Trans. 2007 Jun;35(Pt 3):574-6. doi: 10.1042/BST0350574. Biochem Soc Trans. 2007. PMID: 17511655 Review.
Cited by
- Selective blockade of trypanosomatid protein synthesis by a recombinant antibody anti-Trypanosoma cruzi P2β protein.
Ayub MJ, Nyambega B, Simonetti L, Duffy T, Longhi SA, Gómez KA, Hoebeke J, Levin MJ, Smulski CR. Ayub MJ, et al. PLoS One. 2012;7(5):e36233. doi: 10.1371/journal.pone.0036233. Epub 2012 May 3. PLoS One. 2012. PMID: 22570698 Free PMC article. - Targeting antibodies to the cytoplasm.
Marschall AL, Frenzel A, Schirrmann T, Schüngel M, Dübel S. Marschall AL, et al. MAbs. 2011 Jan-Feb;3(1):3-16. doi: 10.4161/mabs.3.1.14110. Epub 2011 Jan 1. MAbs. 2011. PMID: 21099369 Free PMC article. Review. - Selective targeting of proteins within secretory pathway for endoplasmic reticulum-associated degradation.
Vecchi L, Petris G, Bestagno M, Burrone OR. Vecchi L, et al. J Biol Chem. 2012 Jun 8;287(24):20007-15. doi: 10.1074/jbc.M112.355107. Epub 2012 Apr 20. J Biol Chem. 2012. PMID: 22523070 Free PMC article. - Division of labor among oxidoreductases: TMX1 preferentially acts on transmembrane polypeptides.
Pisoni GB, Ruddock LW, Bulleid N, Molinari M. Pisoni GB, et al. Mol Biol Cell. 2015 Oct 1;26(19):3390-400. doi: 10.1091/mbc.E15-05-0321. Epub 2015 Aug 5. Mol Biol Cell. 2015. PMID: 26246604 Free PMC article. - Current concepts in therapeutic strategies targeting cognitive decline and disease modification in Alzheimer's disease.
Jacobsen JS, Reinhart P, Pangalos MN. Jacobsen JS, et al. NeuroRx. 2005 Oct;2(4):612-26. doi: 10.1602/neurorx.2.4.612. NeuroRx. 2005. PMID: 16489369 Free PMC article. Review.
References
- Bird, R.E., K.D. Hardman, J.W. Jacobson, S. Johnson, B.M. Kaufman, S.M. Lee, T. Lee, S.H. Pope, G.S. Riordan, and M. Whitlow. 1988. Single-chain antigen-binding proteins. Science. 242:423–426. - PubMed
- Cummings, J.L. 2004. Alzheimer's disease. N. Engl. J. Med. 351:56–67. - PubMed
- Dodart, J.C., K.R. Bales, K.S. Gannon, S.J. Greene, R.B. DeMattos, C. Mathis, C.A. DeLong, S. Wu, X. Wu, D.M. Holtzman, and S.M. Paul. 2002. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat. Neurosci. 5:452–457. - PubMed
- Dodel, R.C., H. Hampel, and Y. Du. 2003. Immunotherapy for Alzheimer's disease. Lancet Neurol. 2:215–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical